1. Background
Hyaline cartilage plays a crucial role during normal skeletal development [1]. Transient hyaline cartilage forms the initial models of bones comprising the appendicular, axial skeletons and permanent articular cartilage in joints [2, 3].
Articular cartilage damage is major health problem in the world. Cartilage defects may be due to trauma, mechanical stress, or genetic factors, all of which can lead to osteoarthritis of the joint [4, 5]. However, cartilage has a limited self-repair capacity due to avascularity and lack of innervation. Current treatments such as surgery or implantation of autologous chondrocytes [6-9] are not capable to restore hyaline cartilage in joints.
Mesenchymal stem cells (MSCs) have a multilineage differentiation capacity into cells such as adipocytes, osteoblasts, and chondroblasts [9, 10]. MSCs have been isolated from several tissues including bone marrow, adipose tissue, muscles, deciduous teeth, umbilical cord blood, synovium, brain, blood cells and vessels [11]. Bone marrow is a source frequently used for mesenchymal stem cells [12]. Adult human Adipose stem cells (ASC) can be harvested readily, safely, and abundance by modern liposuction techniques [13-15].
Mesenchymal stem cells (MSCs) have chondrogenic potential with unwanted hypertrophic tendency under transforming growth factor-β (TGFβ) supplemented media [16]. In fetus, during endochondral bone formation TGFβ superfamily, parathyroid hormone-like peptide (PTHrP) and Indian hedgehog (Ihh)) regulate chondrogenesis and the onset of hypertrophy [17-19]. Studies showed that in vitro MSCs chondrogenesis protocols may result in expression of hyaline cartilage components associated with early onset of hypertrophic markers such as collagen type X and alkaline phosphatase [20-23]. MSCs that were differentiated by common in vitro protocols and transplanted to ectopic sites underwent alterations related to endochondral ossification instead of a stable chondrogenic phenotype [24]. In recent years huge efforts have been done to generate hyaline cartilage constructs in in vitro that structurally and functionally mimic normal in vivo permanent hyaline cartilage [25]. So far the inhibition of hypertrophic differentiation of MSC during chondrogenic induction has not been studied interventionally [26].
During joint formation of embryo, mechanical factors play important role in permanent cartilage formation [27]. Different types of mechanical loading were studied on in vitro chondrogenesis [28, 29]. Among mechanical strains, Pulsed ultrasound (LIPUS) has chondrogenic effect on chondrocytes and MSCs in in vitro and in vivo and these studies showed positive effect of LIPUS on extacelluar matix production [30-32]. Ultrasound waves in body tissues can result in micromechanical events at the cellular level [33]. As ultrasound has some advantages such as low-cost, safe in comparison to other mechanical forces and also ultrasound is easy to apply for possible future clinical applications [34], we applied LIUS for inducing chondrogenesis in ASC. We showed that LIUS as a physical inducer work better than TGFβ, in differentiating adipose tissue adult stem cells into chondrocytes by expression of collagen type 2 and aggrecan [35].
2. Objectives
Our hypothesis is application of LIUS on MSC may lead to obtain stable chondrocytes phenotype.
3. Materials and Methods
3.1. ASCs Isolation and Culture
Informed consent and local ethical committee approval was obtained for the use of adipose tissue specimens for this research. ASCs were isolated from subcutaneous adipose tissue that was harvested from female patients (21 - 38 years) undergoing abdomen elective surgical procedures. All experiments were performed on three sets of samples.
Cells were isolated from adipose tissue and characterized using methods previously described with minor modifications [36]. Briefly, the obtained tissue was washed with phosphate-buffered saline (PBS) to remove red blood cells, chopped into small pieces of about 25 - 50 mm3, and the extracellular matrix was digested for 60 minutes at 37°C with collagenase I (0.5 mg/mL) for each gram of adipose tissue (Sigma, St. Louis, MO) in PBS. The ASC-containing cell suspension was centrifuged at 600 g, and the pellet was resuspended in culture medium, which was composed of Dulbecco’s modified Eagle’s medium (DMEM, Sigma) supplemented with 500 µg/mL streptomycin sulfate (Sigma), 600 Lg/mL penicillin (Sigma), and 10% placental human serum [11]. Cultures were washed with PBS buffer after 24 hour plating to remove unattached cells and erythrocytes, and then re-fed with fresh medium [11, 37]. Cultures were maintained at 37°C with 5% CO2 and fed two times per week. ASCs were characterized as we reported previously [11]. We also in our previous work, studied viability and growth rate of cells [11].
For pellet culture, ASCs were differentiated in 15 mL Falcon tubes. ASC pellets were formed by centrifuging 2 × 105 cells at 500 × g in serum-free basal chondrogenic medium consisting of high glucose DMEM (DMEM-HG; Gibco), 10 - 7 M dexamethasone (Sigma, St. Louis, MO), 200 μM ascorbic acid 2-phosphate (Sigma), 1% BSA (sigma), ITS (Gibco), and 1% streptomycin sulfate (Sigma)/penicillin (Sigma). Four groups (control, ultrasound, TGFβ and ultrasound/TGFβ) of pellets were cultured in basal chondrogenic medium for 14 days. In this study for adjusting experiment, TGF-β3 (10 ng/mL) was added to basal chondrogenic medium for TGFβ containing cultures.
3.2. Low-Intensity Ultrasound (LIUS) Treatment
The low intensity ultrasound (LIUS) device (Novin, Iran) with continuous wave at 1 MHz and intensity of 200 mW/cm2 was applied for 10 minutes per day in ultrasound cultures [35, 38]. The distance between transducer and cultures was determined by the Sarvazyan method [39-41].
3.3. Gene Expression
RNA samples were prepared after chondrogenic differentiation. Pellets were disrupted in liquid nitrogen using a small pestle and then RNA isolated using TRIzol reagents (Invitrogen). One microgram of poly (A) + RNA was reverse transcribed to cDNA using RevertAid First Strand cDNA Synthesis Kit (Fermentase) with random hexamer primers. The real-time polymerase chain reaction was performed using SYBR Green kit (ABI, USA) and the Choromo 4 quantitative Real time RT PCR detection System (BioRad). Undiluted cDNA (2 μL) was used in 20 μL PCR mix. Relative gene expression of collagen type X, alkaline phosphatase, Runx2 and Runx2 type II of control and treatment cultures were determined and normalized to housekeeping gene expression (18S) and then compared with untreated (day 0) ASCs. Primers were designed for each gene using the Beacon Designer 7 software (Premier Biosoft International, Palo Alto, CA, USA). Primers used in Real-time RT PCR are listed in Table 1. The triplicate expression level of each target gene was calculated as 2-ΔΔCt, as previously described [42].
| Primer | Primer Sequence | Bp |
|---|---|---|
| 18S | 153 | |
| R | 5′- GTAACCCGTTGAACCCCATT- 3′ | |
| F | 5′- CCATCCAATCGGTAGTAGCG 3′ | |
| Col10 | 225 | |
| F | 5′ –CACTACCCAACACCAAGACA- 3′ | |
| R | 5′- CTGGTTTCCCTACAGCTGAT- 3′ | |
| ALP | 197 | |
| F | 5′- ATGAGGCGGTGGAGATGG- 3′ | |
| R | 5′- CATACAGGATGGCAGTGAAGG- 3′ | |
| Runx2 | 165 | |
| F | 5′- CCGTCCATCCACTCTACCACC- 3′ | |
| R | 5′- AGGCAGAAGTCAGAGGTG- 3′ | |
| Runx2II | 156 | |
| F | 5′- ATGCTTCATTCGCCTCAC- 3′ | |
| R | 5′- ACTGCTTGCAGCCTTAAAT- 3′ |
The Primers is Used for Real-Time RT PCR in This Study
3.4. Histological and Immunohistochemical Analysis
Pellets were harvested and fixed in 10% buffered formalin for 2 hour at room temperature. The fixed pellets, after tissue processing were embedded in paraffin. For immunohistochemical assessment, sections after post fixation with acetone for 5 minutes were washed in PBS (PH 7.5) for 30 minutes at 37°C and then peroxidase activity was blocked by 30 minutes incubation in 0.3% H2O2 in ethanol. After two 5- minutes rinses with PBS the pellets were pretreated with 1mg pepsin (Sigma) (1 mL in of 0.5 M acetic acid) for 40 minutes at 37°C for optimal antigen retrieval and then were washed 5- minutes with PBS. The procedure followed by overnight incubation at room temperature with the indicated antibodies in PBS containing 0.1% BSA. Primary antibodies to, alkaline phosphatase (Serotec, Kidlington, UK) and Indian hedgehog (Ihh) (Abcam, Cambridge, UK) were used (1 to 100).
All incubations were performed in a humidified chamber. After extensive washing with PBS to remove residual primary antibody, reactivity was detected using secondary antibody (horse raddish proxidase, DAKO Cytomation) for 60 minutes at room temperature. Peroxidase activity was visualized by diaminobenzidine) DAB (as substrate with 10 minutes incubation (DAB; DAKO cytomation). The reaction was stopped by rinsing water and the sections were counterstained with Hemotoxylin (Merck). The sections were dehydrated through graded alcohols, cleared with xylene, and permanently mounted.
Data were obtained from three independent donors. For statistical analysis, the data were presented as Means ± SEM. Significances were tested by one way ANOVA (Tukey’s) in Graph pad prism program. P < 0.05 was considered to be statistically significant.
4. Results
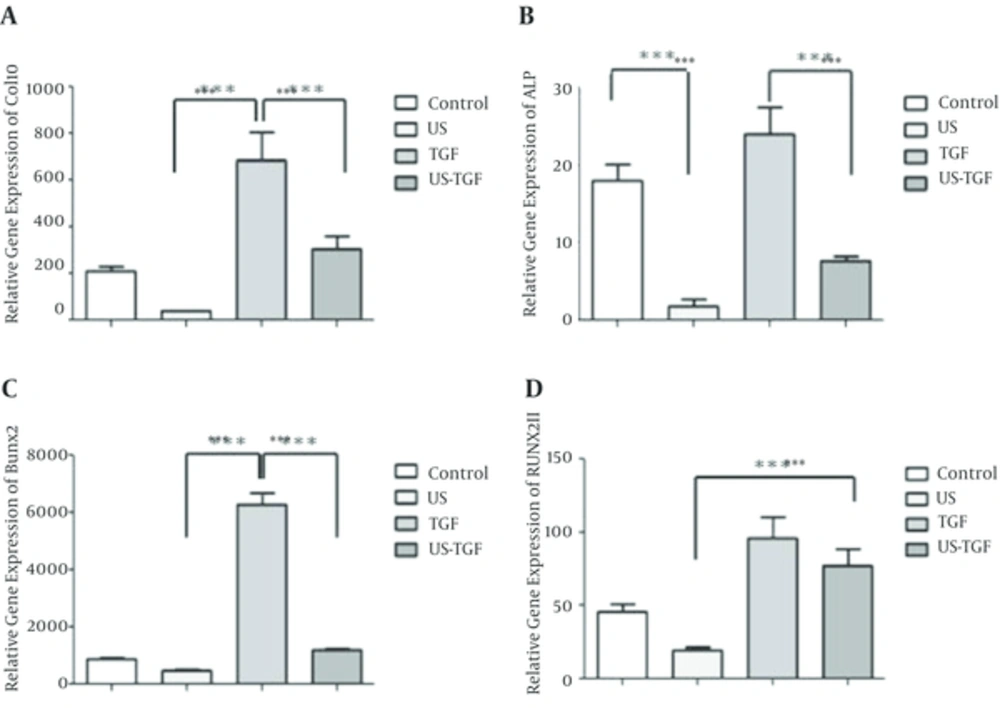
The gene expression of collagen type X, alkaline phosphatase, Runx2 and Runx2 type II was determined by real-time RT PCR of cultured ASCs and differentiated ASCs into chondrocytes with TGFβ and LIUS (Figure 1A - D). The relative gene expression of hypertrophic markers (collagen type X, ALP, Runx2 and Runx2 type II) in TGFβ treated cultures, significantly up-regulated by 685 ± 1 (Figure 1A), 24 ± 3 (Figure 1B), 6280 ± 398 (Figure 1C) and 96 ± 14 (Figure 1D) respectively. The relative expression of collagen type X, ALP, Runx2 and Runx2 type II in ultrasound treated cultures were 39 ± 3 (Figure 1A), 1.7 ± 0.8 (Figure 1B), 481 ± 3 (Figure 1C) and 20 ±2 (Figure 1D) respectively. Also addition ultrasound to TGFβ reduced the expression of collagen type X, ALP, Runx2 and Runx2 type II genes by 2.2 (Figure 1A), 3.1 (Figure 1B), 5.2 (Figure 1C) and 1.2 (Figure 1D) fold respectively. The values of hypertrophic gene in LIUS treated cultures were significantly lower than TGFβ containing cultures (P < 0.001).
The cells were cultured for up to 14 days in four group’s control, LIUS, TGFβ and LIUS-TGFβ. In untreated and treated cultures the relative mRNA expression of hypertrophic chondrogenic markers to 18S housekeeping gene were determined using real-time RT PCR. The relative gene expressions for hypertrophic markers: Col10 A, Alkaline phosphatase; B, Runx2; C and Runx2II D have been shown. Values are Mean ± SEM, n = 3. *** Means is P < 0.001. TGFβ, transforming growth factor-β; TGFβ concentration: 10 ng/mL; LIUS, low-intensity ultrasound. LIUS dose: 10 min/day; Col X, collagen type X; ALP, alkaline phosphatase; Runx2; RunxII, runt-related transcription factor-2 type
Moreover these data show that ultrasound can significantly decrease hypertrophic gene expression effects of TGF containing medium on ASCs (Table 2).
| Analyzed | Control vs US | Control vs TGF | Control vs US-TGF | US vs TGF | US vs US-TGF | TGF vs US-TGF | R2 |
|---|---|---|---|---|---|---|---|
| Col10 | ns | *** | ns | *** | * | ** | 0.56 |
| ALP | *** | ns | ** | *** | ns | *** | 0.68 |
| Runx2 | ns | *** | ns | *** | ns | *** | 0.9 |
| Runx2II | ns | ** | ns | *** | *** | ns | 0.58 |
Statistical Results Summary of Gene Expressions of Col10, ALP, Runx2, Runx2II in Control and Treatment Groupsa
4.1. Immunohistochemistry Results
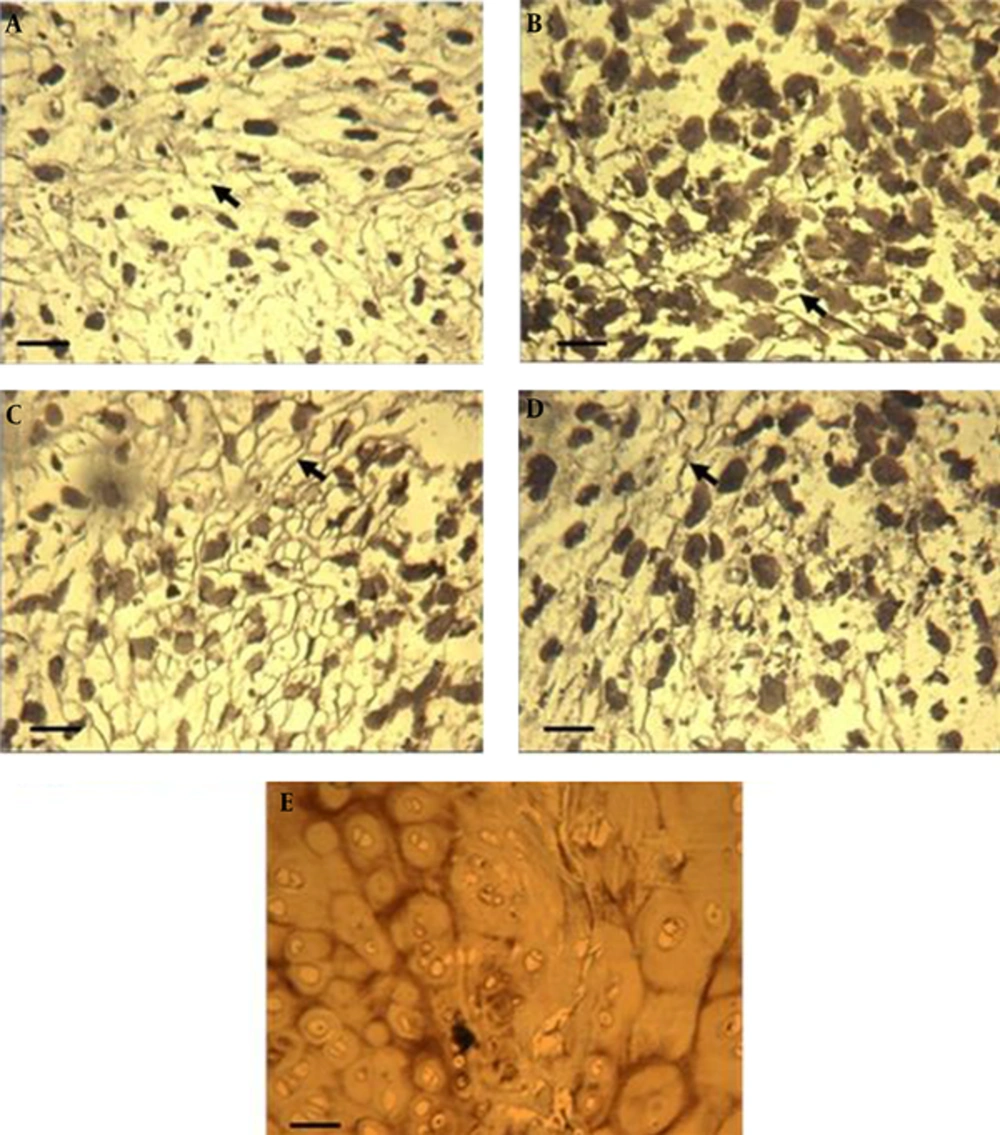
The effects of ultrasound, TGFβ and combination of them on hypertrophic proteins production is evaluated by alkaline phosphatase (ALP) and Ihh immunostaining. Control cultures expressed some ALP protein (Figure 2A). Interestingly LIUS treatment eliminated expression of ALP (Figure 2B) as compared to the control cultures. In LIUS-TGF treated cultures (Figure 2D) versus TGFβ treated cultures as well. Strong protein expression of ALP was found in pellets that did not receive LIUS (Figure 2A and C). TGFβ containing cultures showed more ALP positive area in inset micrographs revealed differences of treated cultures with control cultures. In natural cartilage from costal cartilage was positive for ALP (Figure 2E).
Alkaline phosphatase (ALP) staining of ASCs pellets after 14 days of culture and hyaline cartilage. A, control pellets; B, cultures treated with low-intensity ultrasound, C, TGFβ treated pellet cultures; D, low-intensity ultrasound stimulation plus TGFβ. scale bar: 50 µm; Arrows show ALP proteins in insets, Native human costal cartilage was used as the positive control (E) scale bar: 200 µm. TGFβ, transforming growth factor-β; TGFβ concentration: 10 ng/mL; LIUS, low-intensity ultrasound; LIUS dose: 10 min/day.
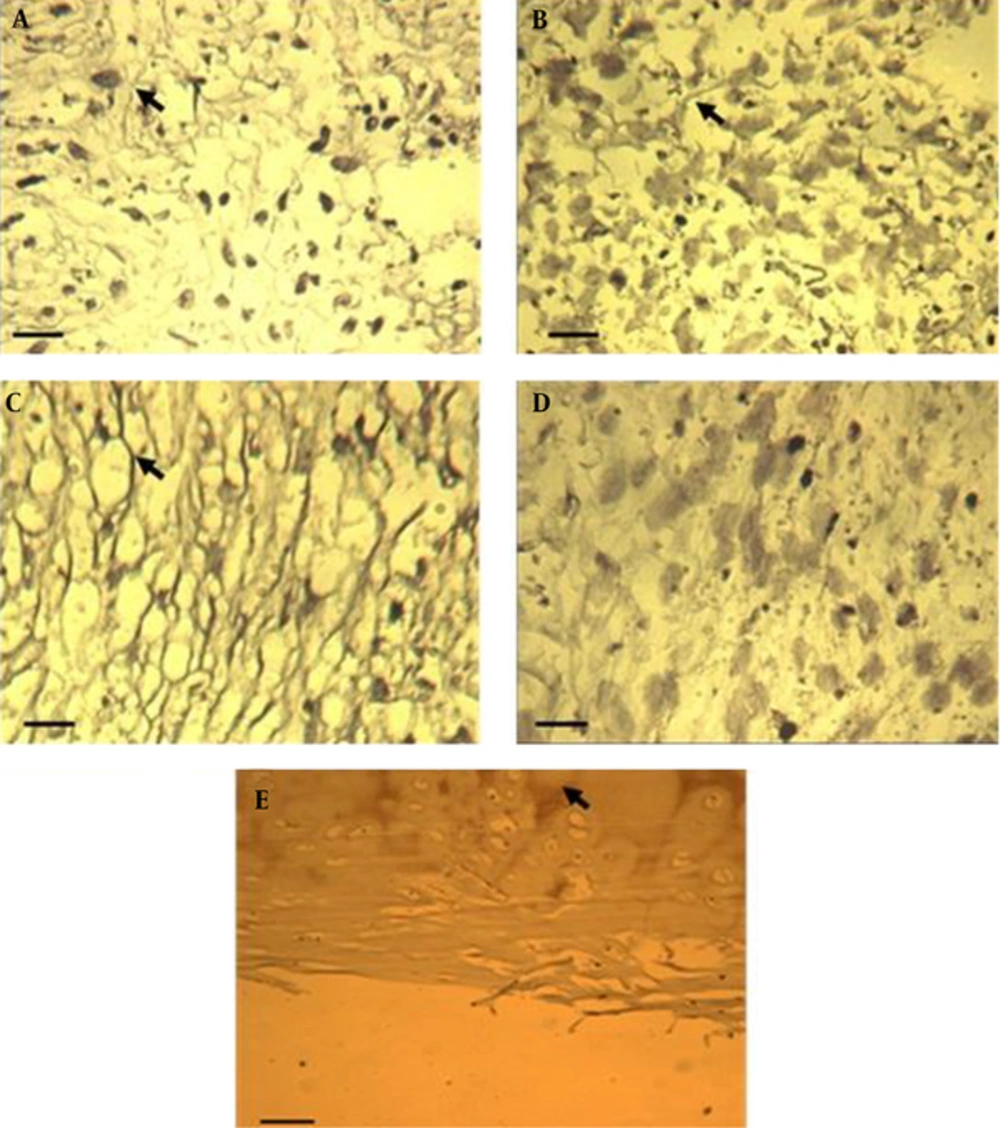
In the staining of Ihh protein, we observed immunopositive area in control and TGF containing cultures (Figure 3A and C). The results of this study showed that Ihh protein is expressed in LIUS and LIUS-TGFβ treated cultures (Figure 3B and D) versus control and TGFβ treated cultures (Figure 3A and C) respectively. In human costal cartilage Ihh protein was observed in endochondrol ossification areas (Figure 3E).
A, Control cultures; B, cultures treated with low-intensity ultrasound; C, TGFβ treated pellets; D, low-intensity ultrasound in combination with TGFβ; E, hyaline cartilage as a positve. Control. Arrows show Ihh proteins around lacunas in treated cultures and hyaline cartilage. Scale bar for untreted and treated pellet cultures with LIUS, TGFβ and LIUS-TGFβ: 50 µm. Scale bar for hyaline cartilage: 200 µm. TGFβ, transforming growth factor-β; TGFβ concentration: 10 ng/mL; LIUS, low-intensity ultrasound; LIUS dose: 10 min/day.
5. Discussion
Tissue engineering approaches for reconstruction of the cartilage is a challenging problem in orthopedic surgery. Mesenchymal stem cells (MSCs) are currently evaluated for possible usage in this area. Many studies have been done to optimize protocols for generation of stable chondrocytes without hypertrophy for transplantation purposes [16]. The aim of the current study was formulation of an innovative physical approach for chondrogenic differentiation instead of chemical TGFβ. Both induction methods (LIUS and TGFβ) capable to induce differentiation of ASCs to chondrocytes by expressing collagen type II proteins. Inhibition of chondrocyte maturation results of this study confirms our pervious collagen type II protein expression results. LIUS induces production of early stage extracelluar matrix of cartilage and more collagen type IIA than collagen type IIB [35]. In cartilage development collagen type IIA is the splice variant of type II collagen that has been found in prechondrocytes and immature chondrocytes [43]. It seems that the splicing protein TASR-1 attributes in removing exon 2 from Col2A [44]. Probably ultrasound mediated these effects via integrins and activativation of MAPK signaling and phosphorylation pathway [45]. Previous studies showed that phosphorylation modulates splicing proteins function [46] and stress stimulus resulted in changes splicing factors [46-49]. Comparison of gene and protein expression results of TGFβ treated cultures showed that TGFβ treated constructs are mature cartilage tissue than LIUS treated constructs. In fact it seems that LIUS differentiated constructs are in early stage of chondrogenesis than TGF constructs. It is possible ultrasound decreases hypertrophic markers of chondrogenesis by decreasing of TGFβ1 and TGFβ2 [50].
In skeletogenesis isoforms of Runx2 express differently in permanent cartilage. Runx2I was highly expressed in osteoprogenitor cells and active osteoblasts [51]. Moreover in Runx-/- mice intramembranous and endochondral ossification is blocked [52]. These data were in accordance with our results that the expression of total Runx2 was too high in TGF cultures than LIUS cultures of present study. While relative gene expression of Runx2II in all cultures about 50 times lower than total Runx2 and therefore amount of Runx2II inhibition by LIUS is not comparable with relative gene expression Runx2.
The results of this study are in agreement with the study that they applied ultrasound parameters close to parameters of our study [38]. LIUS parameters of this study are different from other studies (intensity (30 mW/cm2) and frequency (1.5 MHz) but they showed chondrogenic differentiation without focus on hypertrophic markers evaluations. However complexity of interpretation of results mainly is due to differences in stem cell source, cell culture methods, ultrasound parameters and evaluation methods. In this regard we examined chondrogenic effects of ultrasound in same condition on ASC and BMSC and we observed different behavior of these cells against ultrasound (unpublished data). Previous studies on BMSC showed the effects of ultrasound on chondrogenesis [31]. Zhang et al. reported anatomical origins of MSCs have profound influences on the proliferative and osteogenic capacity of MSCs and BMSC inherently suspected to chondro-osteogenic lineage [53]. Therefore, ASCs from adipose tissue probably might have lower tendencyto osteogenic lineage than BMSC. For evaluation of different ultrasound wave parameters further studies is necessary.
This study focused on studying of hypertrophic genes and proteins after treatment by TGFβ and LIUS. We showed that using TGFβ leads to differentiated chondrocytes with unwanted production of hypertrophic markers. We found that LIUS can down regulate hypertrophic markers such as collagen type X, ALP, Runx2 and Runx2 type II in ASCs and also LIUS could inhibit hypertrophic effects of TGFβ. Recently other study evaluated the prevention of the hypertrophy of differentiated stem cell to chondrocytes and they showed collagens type 2 and X decrease by microgravity [54]. Further investigations are needed to clarify the mechanism of lower stages of LIUS differentiated chondrocytes than TGFβ.
In conclusion, our results indicate LIUS induced chondrocytes are suitable for tissue engineering purposes. This study provides a new vision to cartilage tissue engineering by appropriate use of ultrasound for decreasing of hypertrophic markers especially Ihh protein as intrachondral ossification protein. Therefore, the LIUS induced chondrocytes may have permanent cartilage cell properties. However, it is needed to find out specific marker for permanent cartilage and also to optimize LIUS parameters (distance between ultrasound probe and cells, exposure time, frequency and intensity of waves) in in vitro and in vivo. Moreover continued studies on chondrogenic signaling pathways of ultrasound are vital to understanding its mechanism on chondrogenesis in comparison to TGFβ.