1. Background
Lead (Pb), a common environmental pollutant of worldwide concern, is well-known as a highly toxic agent. It can induce serious adverse health effects including neurological, gastrointestinal, reproductive, renal, hepatic, circulatory and hematological dysfunctions [1]. The currently approved treatment for Pb toxicity is chelation therapy to reduce the burden of the toxic effects of Pb, but the safety and efficacy of the various chelating agents may be questioned [2, 3]. It has been reported that chelating agents may produce a toxic potential themselves and also are incapable of alleviating some toxic effects of lead [3, 4]. Hence, potentially safe alternatives for the treatment of lead poisoning would have very important health-related consequences.
Lead-induced oxidative damage has been proposed as one of the important mechanisms of lead-related pathologies [1]. Several mechanisms have been proposed to mediate the oxidative stress caused by lead, mostly associated with disrupted prooxidant/antioxidant balance. Accumulated evidence of lead’s capacity to induce oxidative stress suggests that lead toxicity may be mitigated by improving the cellular availability of antioxidant agents [1]. It has been proposed that usage of an antioxidant compound in the presence of a chelator will improve the efficacy of the lead poisoning treatment [1, 5]. Hence, it can be presumed that natural compounds with both chelating and antioxidant activities could be good candidates for mitigating adverse effects of lead [2, 5, 6].
Caffeic acid (CA) is a well-known dietary phenolic compound abundantly present in many plants. CA acts as a free-radical scavenger by virtue of its hydrogen-donating ability, forming aryloxyl radicals and stabilization of such radicals by other functional groups in the structure, thus enhancing antioxidant activity [7, 8]. It has been reported that administration of CA protects the liver from nickel induced oxidative damage by decreasing the liver marker enzymes, lipid peroxidative markers and by increasing antioxidant cascade [8]. Moreover, beneficial effect of CA on the improvement of chromium induced oxidative stress in rat intestine has been documented [9].
2. Objectives
In this light, the aim of this study was to evaluate the effects of CA on the lead levels as well as some oxidative status related parameters in blood of mice following experimental lead poisoning.
3. Materials and Methods
3.1. Chemicals
In this experimental study, caffeic acid, lead acetate, 5, 5’-dithiobis-2-nitrobenzoic acid (DTNB), and 2-thiobarbituric acid (TBA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Commercial enzyme kits for superoxide dismutase (Ransod, RANDOX/SD-125) and glutathione peroxidase (Ransel, RANDOX/RS-505) were achieved from Randox Laboratories (UK). The rest of the utilized chemicals were of analytical grade and were supplied by Sigma (St. Lewis, MO, USA) or Merck (Darmstadt, Germany).
Experimental design and sampling: Twenty four male albino mice weighing approximately 30 g were divided randomly into four groups of 6 each. Mice were housed in clean cages at room temperature (22 - 25°C) and a photoperiod of 12 hours light/12 hours dark per day. Animals received standard laboratory balanced commercial diet ad libitum. Group 1 mice received basal diet and tap water throughout the experiment and served as the control. Mice in group 2 received tap water containing 1000 ppm lead acetate. Mice in group 3 received CA (60 mg/kg body weight, daily) i.p. in isotonic saline during lead acetate treatment (1000 ppm in drinking water). Mice in group 4 only received CA (60 mg/kg body weight, daily) i.p. in isotonic saline throughout the experiment. The experiment was approved by Research ethics committee of the faculty of veterinary medicine of Ferdowsi University of Mashhad.
At the end of the experimental period (18 days), blood samples were taken from the heart under ether anesthesia in heparinized vials. The blood samples were centrifuged at 750 g for 20 minutes, and after plasma removal erythrocyte pellet was washed three times with normal saline solution. The washed centrifuged erythrocytes were lysed by adding the same volume of ice-cold redistilled water. Erythrocyte haemolysate were stored at -70°C until analysis.
Biochemical assays: For measurement of Pb levels, sample preparation was done as described previously [10, 11]. Pb concentrations in prepared whole blood samples were determined (in toxicology laboratory of Imam Reza hospital, Mashhad, Iran) by atomic absorption spectrophotometer (Perkin-Elmer 3030) at 283.3 nm wavelength by use of a graphite furnace. Limit of detection for this analysis was 5 ng/g and recovery for spiked samples was > 90%.
Determination of malondialdehyde (MDA) concentration in erythrocyte haemolysate was based on spectrophotometry of the pink colored product of thiobarbituric acid reactive substances, as described by Latha and Pari [12]. The concentration of MDA was calculated using a molar extinction coefficient value of 156,000 M-1 cm-1. Activities of glutathione peroxidase (GPx) and superoxide dismutase (SOD) in erythrocyte haemolysate were measured using Ransel and Ransod kits (Randox Company, UK), respectively, and the results were expressed as units per gram haemoglobin. Hemoglobin concentration was determined by cyanmethaemoglobin method.
Glutathione (GSH) concentration in erythrocyte haemolysate was assayed by the method previously described by Ellaman [13]. In this method, DTNB is reduced to 2-nitro-5’-mercaptobenzoic acid by GSH. The amount of the yellow colour produced was measured at 412 nm and expressed as micromoles per gram hemoglobin.
All experimental values have been represented as mean ± SEM. All results were analyzed using one way analysis of variance (ANOVA), followed by Bonferroni multiple comparisons test. The level of significance was set at P < 0.05. All calculations were performed using SPSS-13 software.
4. Results
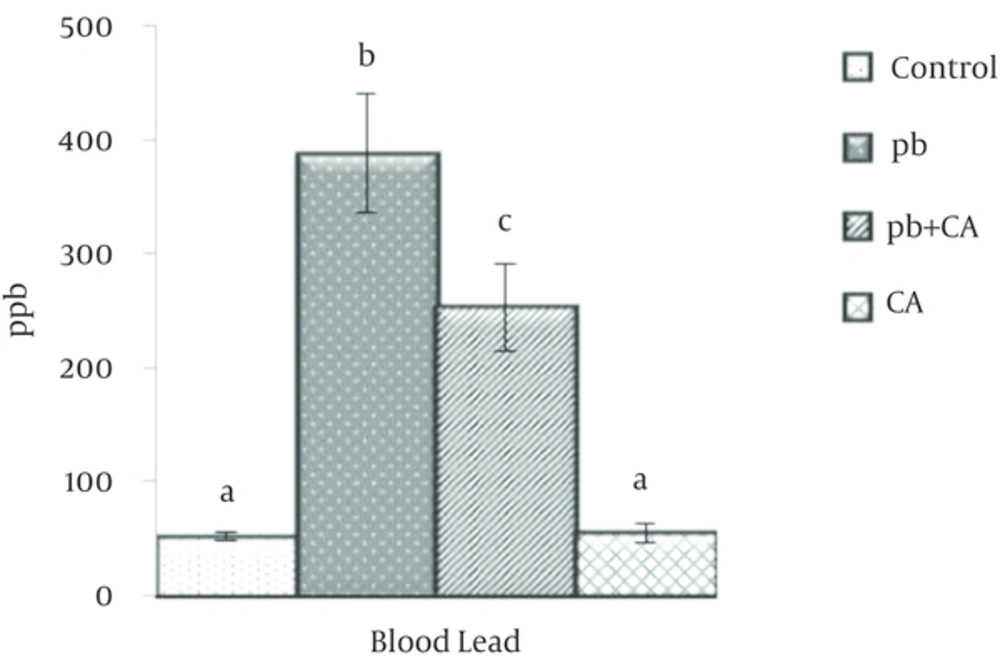
Mortality was not observed in any experimental group throughout the experiment period. Blood Pb concentrations from mice in four treatment groups are compared in Figure 1. Pb concentration increased significantly in group 2 as compared to control unexposed group (P < 0.05). Blood Pb concentration was significantly lower in mice receiving Pb and CA (group 3) as compared to group 2, which received only Pb.
Values of measured oxidative stress-related biomarkers in erythrocyte haemolysate are presented in table 1. Lead acetate exposure caused a significant increase of MDA concentration in erythrocyte haemolysate as compared with control group. Mean erythrocytic SOD and GSH values were decreased in group 2 as compared to control group, although the decrease was only significant for GSH concentration. Administration of CA during Pb exposure increased the attenuated levels of erythrocytic GSH to the levels that were not significantly different from control group. However, CA had no significant effect in decreasing the augmented levels of erythrocytic MDA in group 3.
Abbreviations: MDA, malondialdehyde; GPx, glutathione peroxidase; SOD, superoxide dismutase; GSH, glutathione.
aValues are expressed as mean ± SEM (n = 6 in each group).
bValues in each column with no common superscript differ significantly (P < 0.05).
5. Discussion
In the present study, augmented values of lead concentration in Pb-exposed mice were reduced notably by CA administration. CA ability in forming complexes with some metal ions has been reported previously, the effect which is favored by the presence of two hydroxyl groups attached to its main ring that may produce a site for chelation [8, 9]. Up to authors’ knowledge, CA has not been used as a possible therapeutic approach for lead poisoning. However, it has been proposed that protective mechanism exerted by CA towards nickel toxicity may be due to its ability to chelate the nickel from liver by forming complexes with metal favoring its elimination [8].
Various natural nutrients including methionine, taurine, vitamins B1, B6, C, and E, selenium, copper, calcium, zinc, and alpha-lipoic acid have been shown, in animal studies, to stop or minimize the damaging effects of lead and improve the effects of pharmaceutical chelating agents [1, 14]. In line with the present study finding, some diverse biologically active compounds like allicin [11], methionine [15], thiamine [16], ascorbic acid [17], lipoic acid [18], and pyridoxine [19] has been shown to chelate lead and enhance its excretion from the body, resulting in decreased Pb accumulation in blood and tissues. Moreover, significant efficiency of Smilax glabra extract (contained 26.2% phenolics and 20.3% flavonoids) has been reported in reducing blood and tissue lead burden [20].
An investigation of the literature indicates that oxidative stress is an important contributor to the pathogenesis of lead poisoning, so a synergism might exist between antioxidants and chelating compounds [1]. It is widely accepted that pollutant-induced reactive oxygen species (ROS) generation can initiate or promote lipid peroxidation. Lead-induced enhancement of thiobarbituric acid reactive substances (TBARS) have been reported in some tissues of animals [21-25] that can be due to the generation of ROS without commensurate increases in the level of antioxidant defenses [26]. Results of the present work showed a significant increase of MDA concentrations in the erythrocyte haemolysate of Pb-exposed mice.
Determination of various endogenous antioxidants is applied as a marker of oxidative status. Depletion of tissue GSH is one of the primary factors that permit lipid peroxidation [27]. Results of the present work show significant decrease of GSH concentrations in the erythrocyte haemolysate of lead-exposed mice which is in line with previously published works [23, 28, 29]. Pb+2 binding to sulfhydryl groups can reduce GSH levels and can interfere with its antioxidant activity [18]. However, alterations of SOD and GPx activities were not significant in lead-exposed group in comparison to control group that is different to some extent from previously published results [29, 30].
Beneficial effects of some antioxidant compounds in reducing toxic effects of lead have been reported [30]. Pande et al. [22] demonstrated that lead-induced MDA production was decreased by 30% - 40% after supplementation with antioxidants. It has been also reported that both vitamins C and E are effective in decreasing lead-induced elevated values of TBARS by 60 and 30 percent, respectively [24]. Allicin was markedly effective in decreasing lead-induced lipid peroxidation and increasing the cellular antioxidant enzyme activities and GSH levels [31]. Despite known property of CA as an antioxidant [7, 8], its administration in the present work had no significant effect on increased values of MDA in erythrocyte haemolysate of mice in group 3 as compared to lead-exposed animals in group 2. Despite the present findings, Psotova et al. [32] reported that CA was able to decrease the augmented levels of lipid peroxidation and to eliminate intracellular GSH decline of hepatocytes intoxicated by Fe (III), Cu (II) and tert-butyl hydroperoxide. In addition, CA significantly reduced lipid peroxidation and restored the levels of antioxidant defense in the liver of rats intoxicated with nickel [8].
Like some other antioxidant compounds, phenolic acids, has been also reported to display a bimodal effect depending on their concentration range applied. The results of an in vitro study showed that some natural phenolics including caffeic, chlorogenic, ferulic, protocatechuic and rosmarinic acids behaved as antioxidants with chemoprotective effects in some low concentration ranges [32]. On the other hand, protocatechuic acid at concentrations above 2.5 mM showed in vitro pro-oxidation activity linked to the decrease in reduced glutathione and increase in cellular TBARS [33]. Nakamura et al. [34] described hepatotoxic and nephrotoxic effect of protocatechuic in mice at doses of 500 mg/kg, linked to decrease in GSH. The pro-oxidation effect of CA on Cu2+-induced LDL oxidation has been also described at its high concentrations [35].
The present study is the first investigation in which protective effects of caffeic acid against lead toxicity was evaluated. Some limitations of the present study including lack of sham group receiving intraperitoneal injection and the use of only one dose of caffeic acid should be considered when interpreting the results.
In summary, in the present work some beneficial effects of caffeic acid against Pb toxicity in mice were proved and this compound can be thus proposed as potential prophylactic treatment for amelioration of Pb toxicity. However, the results also indicate that applied dose of caffeic acid was unable to protect against lipid peroxidation induced by lead, despite its antioxidant properties. Although these results seem encouraging, more studies are required to elucidate the molecular basis of the curative properties of caffeic acid, its possible side effects, and its optimal dosage for therapeutic intervention of Pb poisoning.