1. Introduction
Placenta membranacea is scarcely encountered anomaly in which fetal membranes (complete or partial) are covered by functioning villi and the placenta develops as a thin membranous structure occupying the entire periphery of the chorion and placental mass may be often thin and even disrupted [1] for the first time defined by Senesa Sargent, placenta membranacea has already been informed in 1 of 20,000 - 40,000 birth [1, 2]. On the base of latest study in 2013 only 41 cases were reported [3]. This anomaly, pathophysiologically is reasoned by defective differentiate of trophoblastic shell in to the chorion frondosum and chorion leave at 8 - 10 weeks of gestation which result in membranes are covered by chorionic villi [4, 5]. Most of cases prevalently present in the second and third trimesters with of recurrent vaginal bleeding history [2, 6]. Placenta membranacea is accompanied with, preterm delivery, fetal death, intra-uterine growth retardation, post-partum haemorrhage and placental retention [2]. Autosomal recessive polycystic kidney disease (ARPKD) is an important cause of childhood renal- and liver-related morbidity and mortality with variable disease expression. Its major clinical manifestations include ectasia of renal collecting and hepatic biliary ducts and fibrosis of both the liver and kidney [7, 8]. The estimated incidence of ARPKD ranges from 1 in 20,000 to 1 in 40,000 live births [9]. In the present case we reported the first concurrent incidence of two very rare of fetal abnomally ARPKD and placenta membranacea in a fetus.
2. Case Presentation
2.1. Patient
A 25-year-old woman at her first gestation was admitted for inducing abortion with ARPKD in fetus diagnosed in routine sonography fellowship. The age of pregnancy was estimated 16 weeks. Abortion induced with prostaglandin and fetus was studied histologically for ARPKD. During the gross and histological examination of aborted fetus, placenta membranacea was detected in stillbirth.
2.2. Hematological Findings
A decrease in hematocrit, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) was seen. There is not any other abnormality in hematological profile including liver and thyroid enzymes.
2.3. Macroscopic Examination of Aborted Fetus
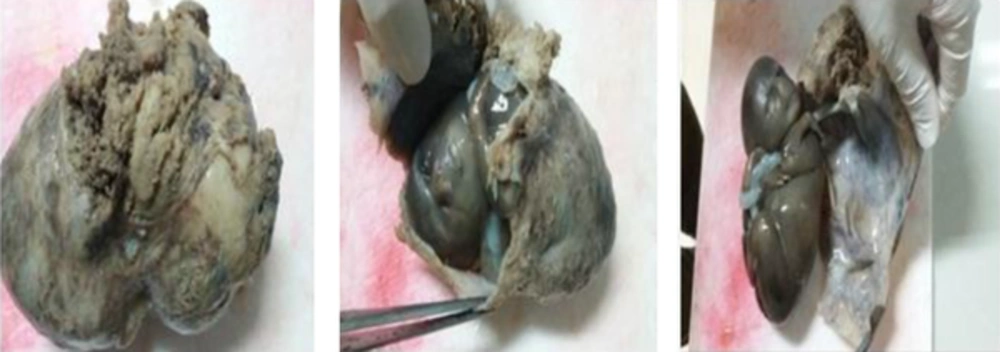
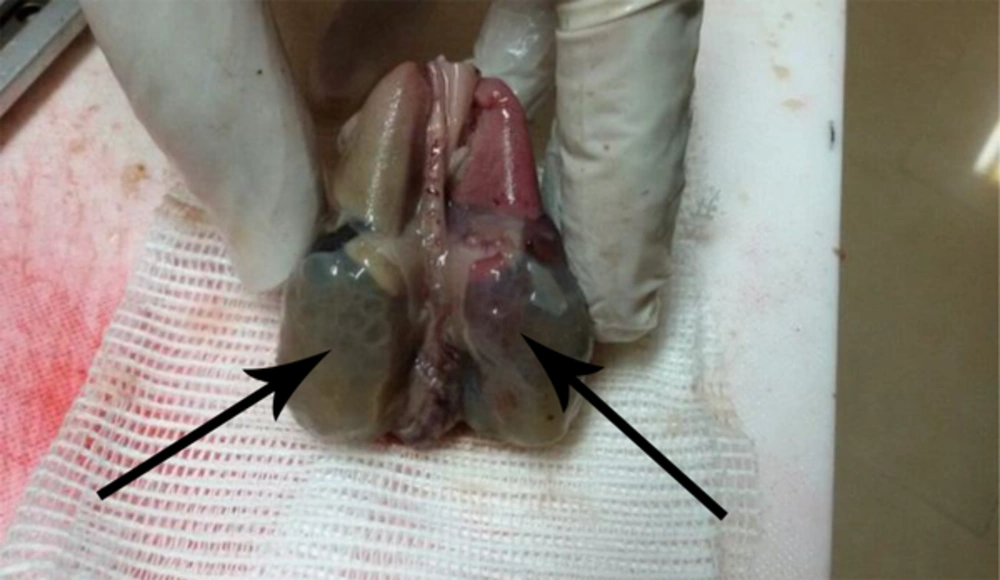
The specimen received in formalin consists of fragment including a placenta totally enveloped the fetus. Inside the membranous placenta is a well preserved female fetus. Crown-rump and crown heel lengths are 120 and 220 mm, weighs 230 g. External feature reveals flat face due to apparently oligohydroamnious (Figure 1). Internal evaluation reveals bilateral enlarged polycystic kidneys (Figure 2). The umbilical cord measuring 28 cm long has two vessels. The umbilical cord indicates no unusual vascular pattern, areas of excessive helical twist, false knot and varix or amniotic adhesion. A series of sections trough the body of placenta show the cut surface to be normally spongy, moist and dark red with no apparent pathologic lesion. Trimmed body of placenta weighs 110 g.
2.4. Microscopic Diagnosis
A well preserved female fetus with Potter phenotype to oligohydramnious (Figure 1).
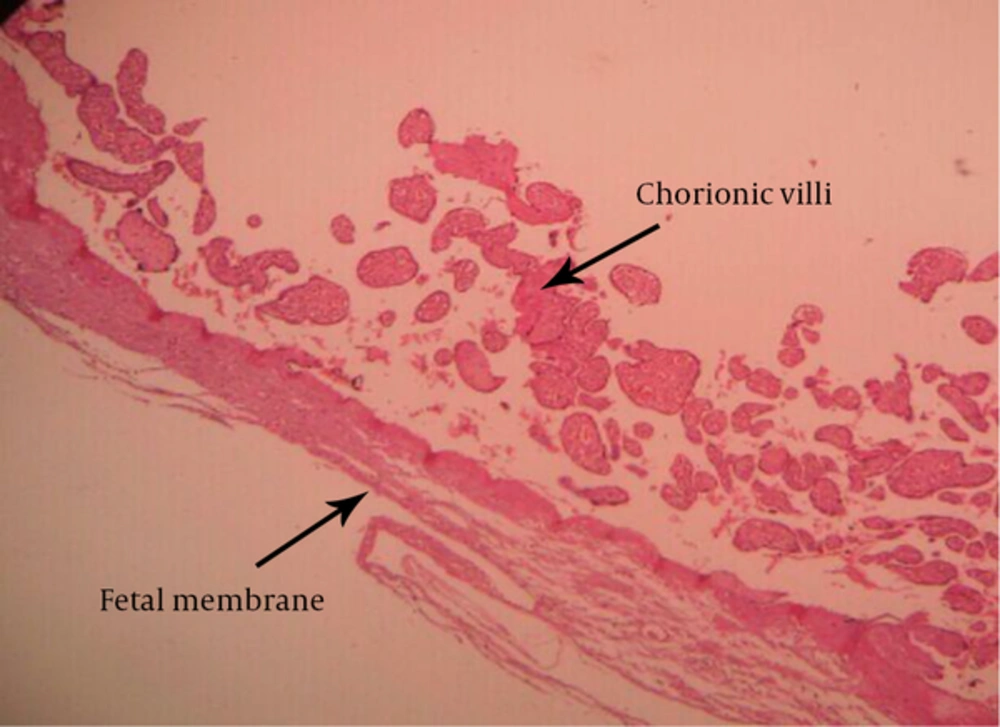
-placental membranacea (Figure 3).
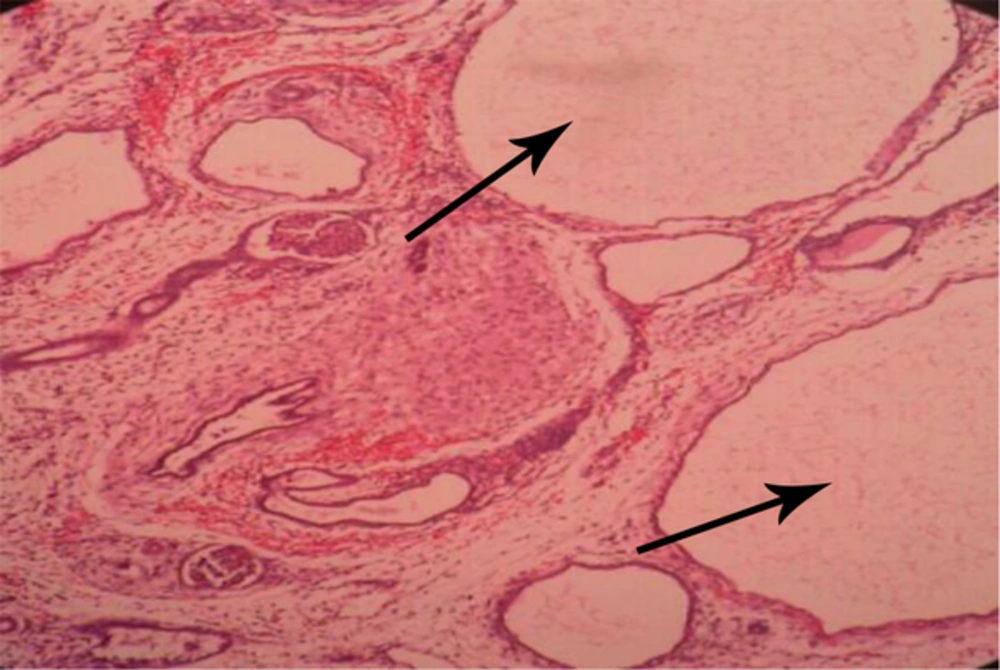
-Autosomal recessive (infantile) polycystic kidney disease. (Figure 4).
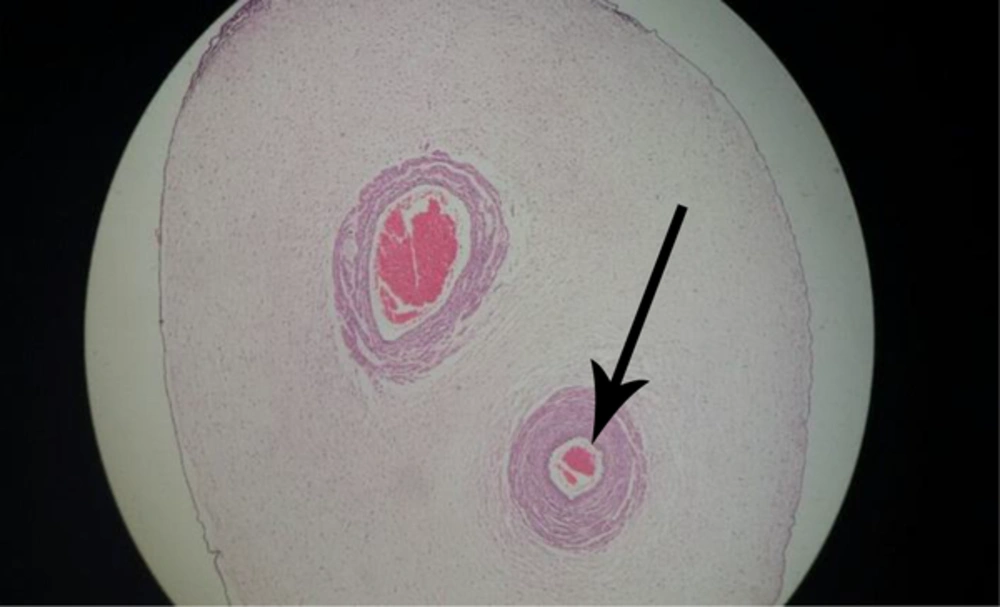
-Single umbilical artery (Figure 5).
3. Discussion
Placentae membranacea take place when chorionic villi are spread all over the amniotic sac as a result of failure of regression in the first trimester [10] and subsequently occurred in second or third trimester of gestation [2, 6]. The etiology is not entirely clarified, but it appears clear that those villi designed to degenerate and produce the chorion laeve are retained while there is lack of growth of the villi ordained to become the chorion frondosum. Underlying reasons postulated for lack of villous growth associate mostly to anomalies of the endometrium such as endometrial hypoplasia, poor vascular supply of the decidua basalis, endometritis, multiple curettages, adenomyosis or atrophy of the endometrium placenta membranacea may manifest clinically as early bleeding and placenta previa [8]. Affected gestations frequently result in premature delivery and placenta accreta is rather prevalent. Spontaneous abortion and second trimester fetal death have also been informed [8]. In this complication placenta have been characterized by tan colored membrane with outspread placental cotyledons [3, 11]. In our case, only one artery was seen in umbilicus, an anomaly was seen with incidence of 1% and fetus kidneys were detected polycystic. We diagnosed an ARPKD in fetus characterized with an incidence of 1: 20,000 live births and is classically recognized in the first few weeks after birth resulting in a 30% death rate in neonates [12]. In ARPKD kidneys retain their shape but are larger than the normal anatomical ranges which are precisely detected in our case. Mutations in the PKHD1 gene on chromosome 6p12, which is among the largest human genes cause ARPKD [13, 14]. Up now there are not found any basic or genetic reason for placentae membranacea [10]. In the present case, for the first time, placentae membranacea and ARPKD occurred in one fetus. As ARPKD is a result of mutation in PKHD1 so our finding may be initiate a new investigation about genetic relation between placenta membranacea and autosomal recessive polycystic kidney disease.