1. Background
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by hyperplasia of synoviocytes leading to joint destruction and permanent deformity. While this disease has a worldwide distribution and increases in prevalence with age, its pathogenesis is not clearly understood [1]. Based on the resemblances between the joint pathology in CIA and RA, it suggests as a relevant murine model to search for new therapeutic agents against arthritis [2]. Although, nowadays therapeutic agents lessen the progression rate of the disease, joint damage typically occurs before patients are diagnosed. Although, the definite causes of RA are ambiguous, some evidence suggests that telomerase is involved in the pathogenesis of this disease [3, 4].
Telomerase is a ribonucleoprotein enzyme which enlarges the ends of chromosomes by adding repeated units of TTAGGG. An RNA component of this enzyme, called hTERT which serves as a template for addition of telomeric repeats. A number of reports have mentioned a relationship between degree of invasiveness and the increased telomerase activity. In an in vitro study, transfection of rheumatoid arthritis synovial fibroblasts with vectors expressing anti-sense oligonucleotide against the hTERT has led to cytolysis of these cells that exhibit high telomerase activity [5].
Green tea prepared from the dried leaves of Camellia sinensis, is drunk widely by people globally. There are many polyphenolic compounds in green tea among them, epigallocatechin gallate (EGCG) should be considered. These antioxidants polyphenols possess anti-inflammatory properties. Fail to adequately control the underlying pathophysiology of RA suggesting that finding new therapeutic agents. In this study we evaluated the effects of Camellia sinensis dried leaves extract on telomerase activity in experimental collagen-induced arthritis.
2. Methods
In this experimental study, sixteen healthy adult male Lewis rats with average body weight (160 - 180 g) were obtained from national institute of genetic engineering and biotechnology (NIGEB, Tehran, Iran). The rats housed in a controlled environment and determined food and water were divided at random into non-treated and treated groups with Camellia sinensis stew (CSS) 1 mg/kg/d. Animal care was in compliance with Iranian regulations on the protection of animals used for experimental and other scientific purposes and in accordance with the international principles for biomedical research involving animals, revised in 1985. Fine grade materials were obtained from Sigma-Aldrich (St. Louis, MO, USA).
For Induction of CIA, Bovine CII was emulsified with an equal volume of Freund’s complete adjuvant (CFA). For induction CIA, on day one, 100 µL of emulsion were injected intradermally at the base of the tail. A second injection of CII in CFA was administered on day 21. The orally administration of CSS were started on day 25 post-immunization and continued until final assessment on day 35 [6]. During this period, clinical examination was taken intermittently. The paws and knees were then removed for histopathological assay. Macroscopic scoring system using a scale from 0 to 4 for each paw was used to evaluate arthritis [7]. On day 35, animals were anesthetized and killed. After fixation and decalcification of paws and knees, they stained with hematoxylin and eosin for histological examination. Joint damage was assessed as previously described [7]. According to the severity of damage, joint erosion was graded on a scale of 0 - 3 for each limb.
For radiography of normal and arthritic hind paws, the rats were anaesthetized with sodium pentobarbital (45 mg kg-1, i.p.). Based on a score from 0 to 3 (score 0, no bone damage; score 1, tissue swelling and edema; score 2, joint erosion; score 3, bone erosion and osteophyte formation) a blinded investigator performed radiograph score.
Biopsies of synovial tissue were obtained aseptically from the knee joints of rat. For harvesting the synovium from the knee joint, the patellar tendon with straight skin incision exposed. After cutting the patellar tendon transversely, and peeling the tendon, the infrapatellar fat pad from the femur and tibia was separated and then the synovium put into PBS in a Falcon tube. Finally, synovial tissue specimens were digested with 0.2% collagenase in high-glucose DMEM plus 10% FBS and antibiotics. Following overnight incubation at 37°C, collected cells were plated and allowed to reach confluency at 37°C in a humidified atmosphere of 5% CO2.
Telomerase activity of was measured by TRAPeze telomerase detection kit (Intergen, Inc., USA) according to the kit instructions. Briefly, using CHAPS lysis buffer, harvested cells from synovial tissue were lysed and for 30 minutes at 30°C the telomerase was first extended and then amplified by 30 cycles of PCR. By silver nitrate staining, the products of PCR were detected. Telomerase activity was calculated as the ratio of the intensity of telomerase ladders to the intensity of the 36-bp internal standard.
All data are expressed as the mean ± SEM. The results of different experiment conditions were compared using independent sample t-test utilizing the SPSS (ver. 16) software, and the results were considered statistically significant at P < 0.05.
3. Results
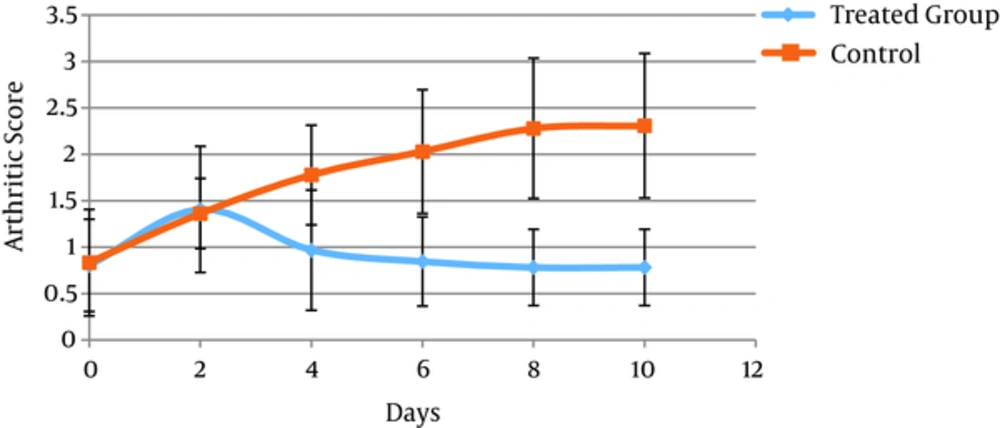
The efficacy of RA therapy with CSS is presented in Figure 1. CIA developed in rat immunized with CII, and clinical signs (periarticular erythema and edema) of the disease first appeared in the hind paws between days 25 and 27 after CII challenge, with a 100% incidence of CIA by day 27. The administration of CSS to arthritic rats could rapidly reverse paw edema. The difference between control group and treated rats on days 30 - 35 of experiment was significant (P < 0.000).
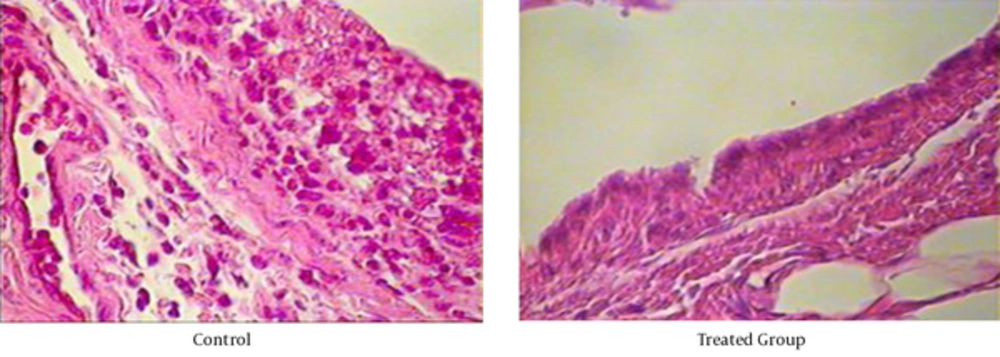
Histological evaluation of the paws in the control animals reveals signs of severe arthritis along with inflammatory cells infiltrate (Figure 2). In treated group, histopathological assessment showed a reduced inflammatory cells infiltrate in the joints of treated rats, as well as markedly reduction in the number of osteoclasts, tissue edema and bone erosion indicated of its effectiveness for ameliorating synovial inflammation and prevention of joints destruction. The results of histopathology score in both treated and non-treated rats were shown in Table 1.
| Variables | Group | Mean | Std. Deviation | t | Sig. (2-tailed) | 95% CI of the Difference | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Arthritis score | Control | 2.4062 | 0.76692 | 5.284 | 0.000 | 0.96537 | 2.28463 |
| Treated group | 0.7812 | 0.41052 | |||||
| Histopathology score | Control | 3.1250 | 0.83452 | 3.771 | 0.002 | 0.86256 | 3.13744 |
| Treated group | 1.1250 | 1.24642 | |||||
| Radiology score | Control | 2.2500 | 0.99103 | 2.144 | 0.05 | 0.00021 | 1.93771 |
| Treated group | 1.2812 | 0.80664 | |||||
| Telomerase activity | Control | 1.0000E2 | 0.95000 | 66.806 | 0.000 | 45.43964 | 49.38036 |
| Treated group | 52.5900 | 0.78000 | |||||
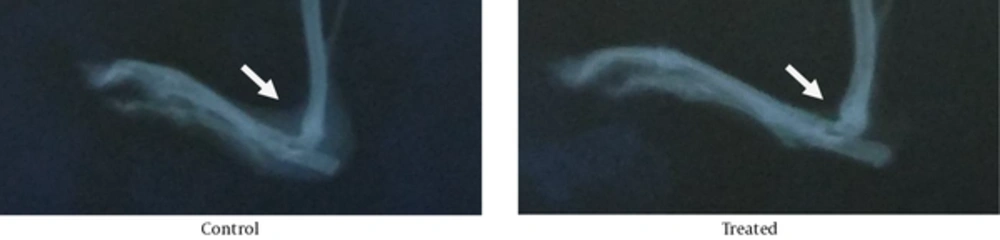
In addition, our radiographic finding didn’t show any statistically differences between treated and non-treated arthritic hind paws (P = 0.05). The mean differences between treated and non-treated arthritic hind paws were 1.2812 + 0.80664 vs. 2.25 + 0.99103, respectively. The X-ray results revealed that CSS-treated rats had more intact joint structure, as compared with their control counterparts (Figure 3).
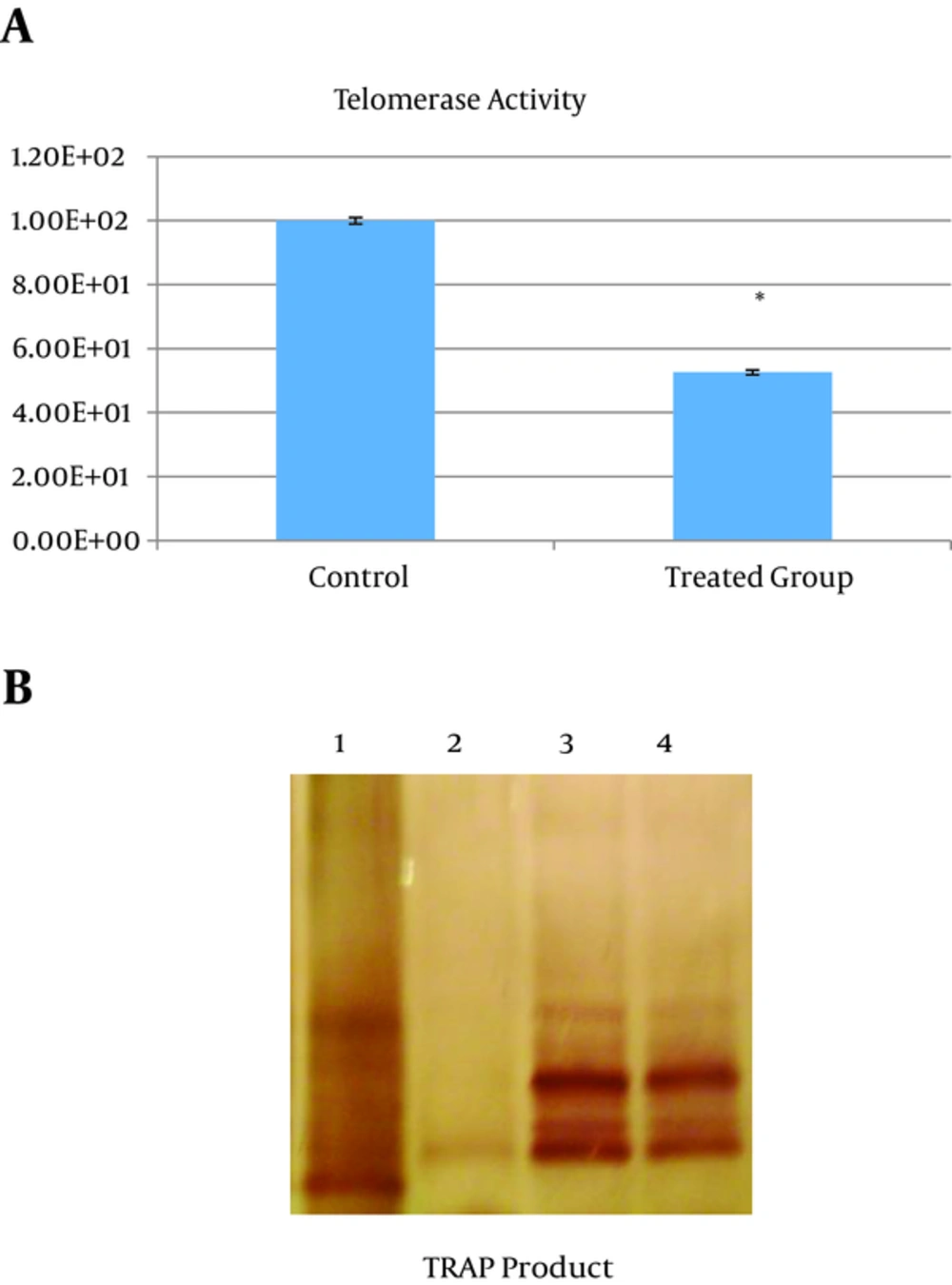
Cells obtained from animal treated with orally administration of Camellia sinensis stew showed a significant reduction of telomerase activity (about 50%) compared with control group; 1.0000 E2 + 0.95000 vs. 52.5900 + 0.78000, respectively (P < 0.000) (Figure 4).
Telomerase Activity of Synovial Cells of Treated and Control Rats (A).A Representative of Gel Electrophoresis for Telomerase Activity Measurement is Shown After the Telomerase in Cells Was Amplified by 30 Cycles of PCR and Stained by Silver Nitrate. Lane 1, Size Marker; Lane 2, Negative Control; Lane Treated Group; Lane 4, Control. (B)
4. Discussion
The recent investigations indicate that rheumatoid arthritis should not be considered as a benign disease. Moreover, it is associated with diminished long term survival. The clinical trials on different treatment strategies in rheumatoid arthritis have shown that various types of medications are useful in the treatment of rheumatic diseases [8-10]. In this investigation, to imitate the clinical scenario of RA, CIA was induced in Lewis rats and subsequently, the anti-arthritic potency of Camellia sinensis stew was assessed in mentioned experimental model. This beverage is being used for many years and possesses antioxidant activities, anti-inflammatory, anti-apoptotic properties and protective effects in autoimmune animal models [11-15].
Here, we demonstrate that treatment with CSS reduces the development of clinical signs and the degree of joint injury in CIA model. Moreover, reduced inflammatory cells infiltrate in the joints of treated animals with type II collagen was occurred due to down-regulation of pro-inflammatory cytokines and chemokine receptor in synovial tissues [16]. All of these findings support the view that CSS attenuates the degree of arthritis and joint damage caused by collagen in this model.
In RA, osteoclasts abnormally activated due to interaction with synovial fibroblasts and helper T cells and, in turn induce bone destruction. In our findings, a reduction in the clinical scores of joint inflammation and joint erosion were in agreement with the result of trial in which the green tea polyphenol epigallocatechin-3-gallateas a potent antioxidant improves autoimmune arthritis by both regulation of TH17 regulatory cells and inhibition of osteoclasts development [17]. Moreover, there is more evidence that polyphenols in green tea, particularly EGCG, inhibit matrix metalloproteinase activity which plays a fundamental role in pathogenesis of many diseases such as RA [18]. Although, joint erosion in CSS-fed animals reduced, the level of significance in the radiological index of the joints in treated rats compared to control could not be achieved. It might be attributed to numerous factors including the number of animals in each group and the delay in treatment schedule.
To investigate the anti-telomerase effect of CSS, we used the synovial fibroblasts. Synovial cells proliferate and transform the normally synovium into an invasive pannus. This architecture creates a hypoxic microenvironment and, in turn, through the increased production of reactive oxygen species induce inflammation [19]. Since, these cells contribute to chronic inflammatory responses in RA. Data obtained from telomerase assay exhibited that CSS induced reduction of telomerase activity compares with control group. Since the regulatory role of telomerase on cell proliferation has been recognized, its inhibition by various molecules has been considered by many investigators as a new approach for cancer therapy. Moreover, the crucial role of telomerase activity in other feature of neoplastic phenotype like invasion, apoptosis resistancy and so on were determined in some cancer models. Based on the presence of two E-box boxes on the hTERT gene promoter which are the binding sites of transcription factors like c-Myc, the activation of telomerase was controlled [20, 21]. Wang et al. [22] found that c-Myc protein level was significantly decreased after EGCG treatment, which was accompanied by the reduction of the telomerase activity. However, molecular mechanisms by which CSS induces reduction of telomerase activity are thus still called for.
In conclusion, we show that CSS effectively suppresses collagen arthritis and, a potent inhibitory effect on telomerase activity and based on its high tolerability, it can be recommended for long-term administration, in the future investigations.