1. Background
Sulfonamides are relatively old synthetic antibacterial compounds, which can inhibit both Gram-positive and Gram-negative bacteria, as well as some protozoa, such as coccidials [1]. Sulfonamides can interfere with multiplication of the bacterial cell by completely competitively binding the para aminobenzoic acid (PABA) and prevent the folic acid formation, an important metabolite in DNA synthesis. Therefore, sulfonamides are usually bacteriostatic [2, 3]. Because of low cost, ease of administration, and a wide range of application caused the extensive use of sulfonamide derivatives and resulting in a rapid rise of bacteria-resistance, cross-resistance among sulfonamides and residues appearing in animal products [1, 4, 5]. The concentrations of residues are varying considerably in various tissues and the highest level exists in body fats and/or in organs that actively metabolize and excrete them especially liver and kidney [5, 6].
In poultry science, sulfonamides have been used for treating of various diseases like cocidiosis, infectious coryza, pullorum disease and flow typhoid [1]. As reported previously, following the treatment of infected hen with sulfonamides, the residues of them are also retained in the chicken and egg layers [7]. Studies revealed that the presence of sulfonamides residues in food is considered harmful to consumers [5]. These have adverse reactions such as renal insufficiency side effects [8, 9].
2. Objectives
In this present study, we investigated the effects of the administration of different doses of sulfadiazine in chicken embryo as an experimental model in order to detect histopathologic alterations between low and high doses. According to the author’s knowledge, there is no information currently available about the sulfonamide-associated nephrotoxicity with possible underlying mechanisms in chicken embryo, and the present study is the first attempt in this field.
3. Materials and Methods
This study was conformed to the rules of the Protection of vertebrate animals used for experimental and other scientific purposes [10]. In this experimental study, one hundred fertile eggs were obtained from a broiler breeder farm (Ross 308 strain). All eggs weighed with an average of 63 ± 1 g were divided into five groups: 1) control group (without injection), 2) group injected with 2 mg sulfadiazine, 3) group injected with 10 mg sulfadiazine, 4) group injected with 30 mg sulfadiazine and 5) group injected with 70 mg sulfadiazine. Then, the eggs were incubated at 37.5°C and 65% relative humidity. On third day of incubation, the eggs were candled, clear eggs and dead embryos were removed for examination. Then shortly after, the eggs were injected in ovo at fourth day of incubation via chorioallantoic rout with 0.2 mL of mentioned doses. The injection site was sealed with Betadine®. To avoid contamination, all injections were carried out in a clean room and all the equipments were sterilized. On the eighteen day of incubation, the fertile eggs were transferred to the hatcher and kept at a temperature of 37°C until they hatch.
During the incubation, dead embryos were removed (n = 9). After hatching, the kidney from the newly hatched chick was taken out (n = 30) and fixed in 10% formalin for histological examination. Some of kidneys (n = 80) were also stored at -70°C until used for assessing oxidative stress examinations. The isolated whole kidney tissue was homogenized with 10 times (w/v) sodium phosphate buffer. The homogenate was centrifuged at 3,000 rpm for 15 minutes, and the supernatant was used for estimation of biochemical indices.
Histopathologic examination: Following histological fixation, the renal tissues were dehydrated by transferring through a series of alcohols with increasing concentrations, placed into xylol and embedded in paraffin. A microtome was used to make 8 cuts that were 6-7 μm and they were stained with hematoxylin-eosin (H & E). The sections were observed under light microscope. Histopathologic changes in renal tissues were graded as follows: (-) showing no changes, (+), (++) and (+++) indicating mild, moderate and severe changes, respectively.
3.1. Measurement of Oxidative Stress Parameters in Organ System
3.1.1. The Ferric Reducing Capacity Assay
The antioxidant capacity of samples was determined by measuring the ability of samples to reduce Fe3+ to Fe2+. The complex between Fe2+ and 2, 4, 6-tris-(2-pyridyl)-1, 3, 5-triazine (TPTZ) gives a blue color with absorbance at 593 nm [11].
3.1.2. Measurement of Reduced Glutathione (GSH)
The glutathione content was applied according to the previous method [12]. Briefly, the cells were rinsed three times with phosphate-buffered saline (PBS). The cell solution mixed with 20% trichloroacetic acid. Samples were centrifuged at 3000 rpm for 15 minutes. The supernatant was mixed with 4 volumes of Tris. Then, 1 mM 5, 5’-dithiobis (2-nitrobenzoic acid) (DTNB) was added to the sample and incubated for 30 minutes. The absorbance was read at 412 nm.
3.1.3. Measurement of Lipid Peroxidation
The formation of thiobarbituric acid in organ samples was assessed for the measurement of lipid peroxidation according to an original method [13]. Briefly, the supernatant of the tissue homogenate was mixed with 20% trichloroacetic acid and the mixture was centrifuged. Then, thiobarbituric acid was added to the supernatant and heated. The absorbance of the supernatant was measured at 532 nm. The values were expressed in nmoles (nM) malodialdehyde (MDA), using a molar extinction coefficient of 1.56 × 105 M-1cm-1.
Oxidative stress data were analyzed using one way ANOVA using SPSS-16 software followed by Tukey-Kramer post-hoc test for multiple comparisons. Kolmogorov Smirnov tests showed that these data were normally distributed. The evaluation was made by the comparison of groups. The results were presented as means ± SEM and P < 0.05 was considered significant.
4. Results
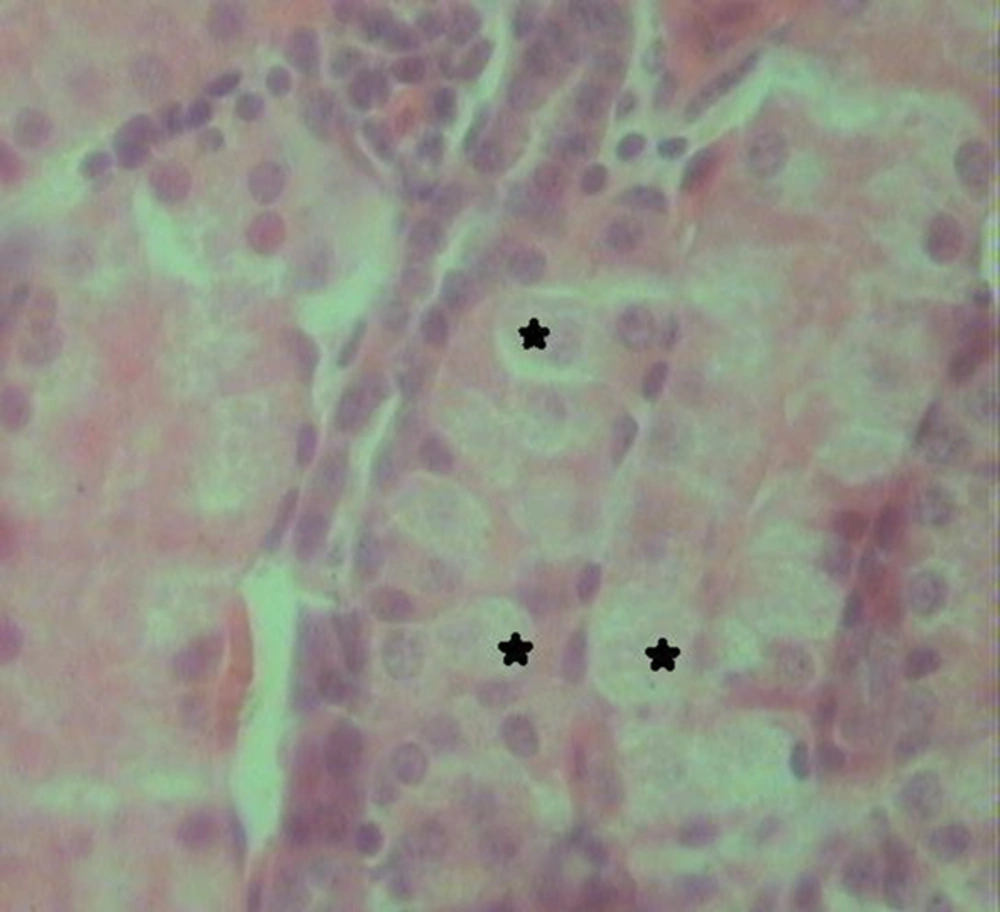
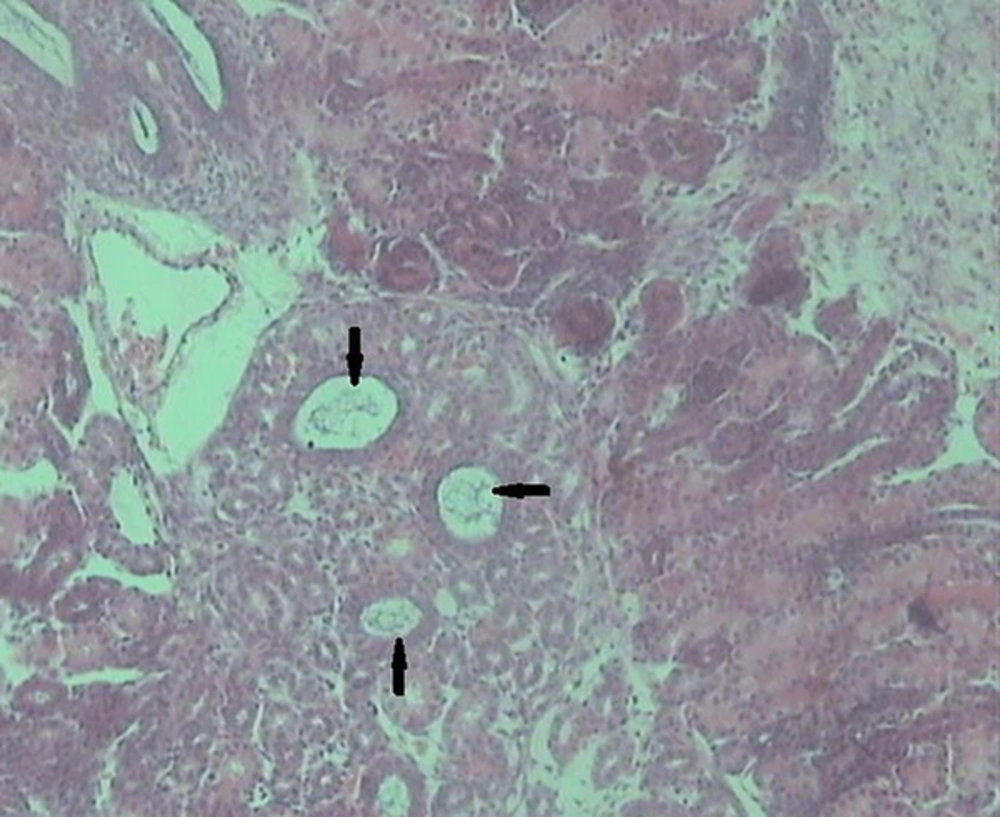
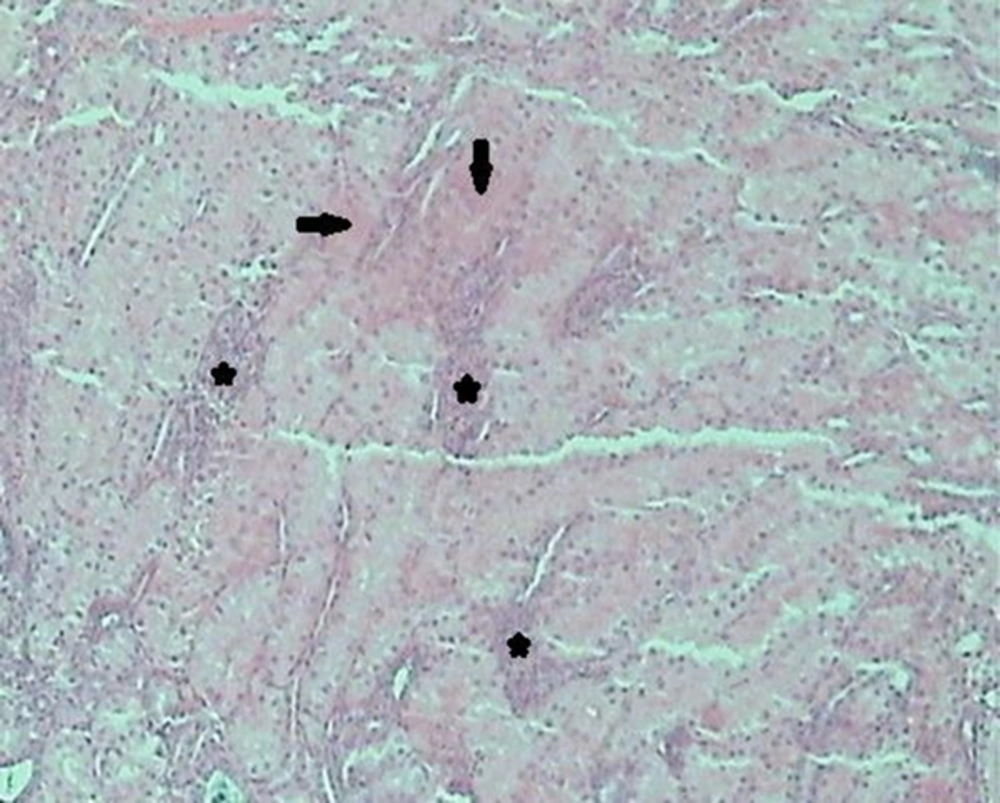
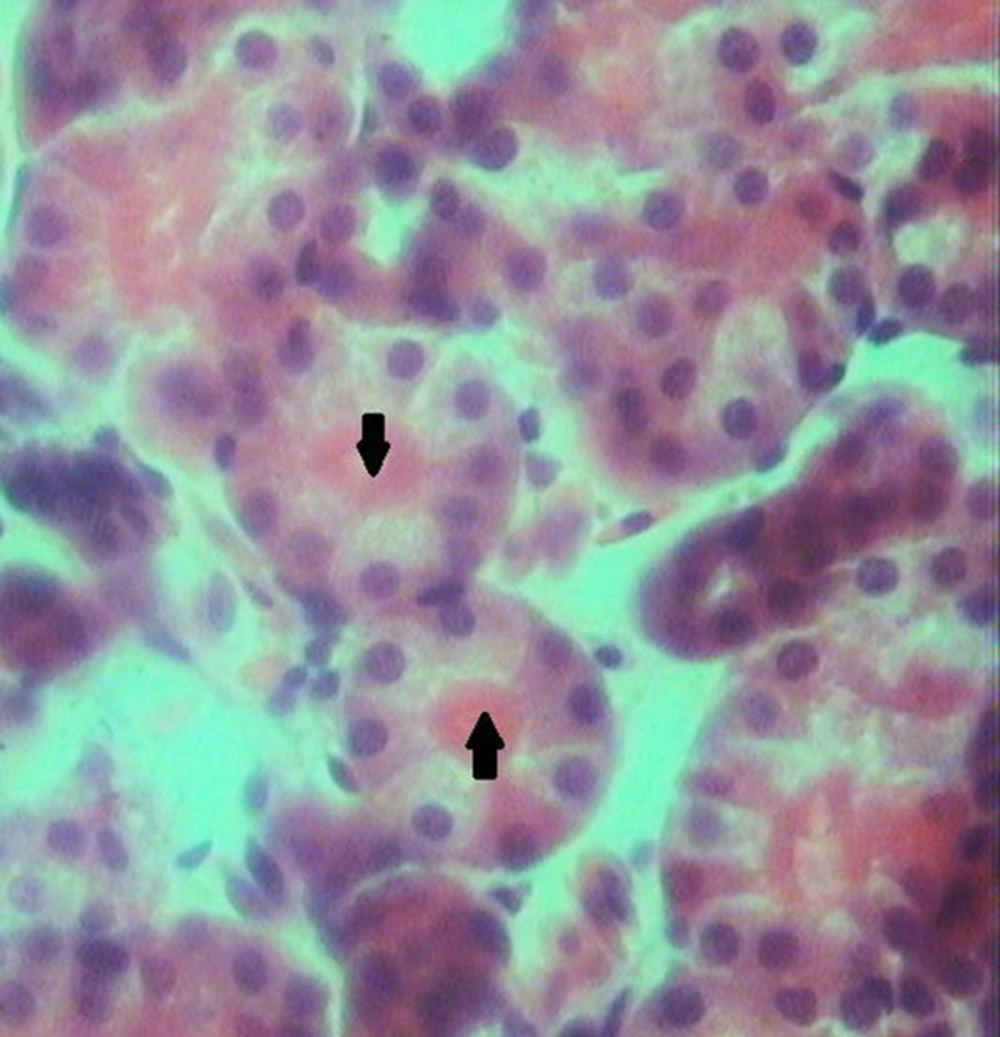
Histological examination of the kidney tissues in the control group revealed no significant deviation from the normal histological structures. In group 2, hydropic degeneration was observed in epithelial cells of the tubules. No evidence of tubular epithelial necrosis was seen. Within the lumen of collecting tubules, fan-shaped, empty spaces indicated sulfadiazine crystals. In addition, hyaline casts were seen in the lumen of some tubules. In general, pathological changes, formed by administration of 2 mg of sulfadiazine, were mild (Table 1). In group 3, the pathological changes compared with those in the group 2 were very common. However, hydropic degeneration (Figure 1) was more severe than group 2 and also, some apoptotic epithelial cells, mild multifocal non-supportive interstitial nephritis and congestion has been found in this group (Table 1). Fan-shaped, empty spaces representing the spaces once occupied by sulfadiazine crystals had been often present (Figure 2). Hyaline casts were seen in the lumen of some tubules. In group 4, hydropic degeneration was more severe compared to previous groups. Fragmentation and shedding of tubular epithelium that indicated cellular necrosis were significantly identified in this group. Some Bowman’s spaces and tubules showed dilation. The severity of interstitial nephritis was more than group 3. Congestion was more severe compared to previous groups (Table 1), and also, some foci of hemorrhage were observed in the renal interstitium. In group 5, epithelial necrosis was more severe compared to group 4. Fan-shaped, empty spaces accumulated in the lumen of collecting tubules. Multifocal interstitial nephritis was reported (Figure 3). Mild fibrosis areas and hyaline cast were observed in the lumen of many tubules (Figure 4). Congestion and hemorrhage were observed in the cortical and medullary interstitial areas. Some glomerulus showed atrophic change and also dilation of Bowman’s spaces (Table 1).
| Groups | |||||
|---|---|---|---|---|---|
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
| - | + | ++ | +++ | +++ | |
| - | - | - | + | +++ | |
| - | - | - | - | + | |
| - | + | ++ | +++ | +++ | |
| - | + | + | ++ | +++ | |
| - | - | + | ++ | +++ | |
| - | - | + | ++ | +++ | |
| - | - | - | - | + | |
Histopathologic Changes Induced by Different Doses of Sulfadiazine in Renal Tissuea
Oxidative stress results have illustrated in Table 2. There was no significant difference between the levels of antioxidant in kidney of group 1and 2 compared to the control group, but in high dose group (4 and 5) antioxidant level was lower than control and other groups, (P = 0.03) for group 4 vs. control group, (P = 0.01) for group 5 vs. control group and (P = 0.02) for group 5 vs. group 2.
| Group 1 (Control) | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| 1.89 ± 0.24 | 1.62 ± 0.17 | 1.37 ± 0.14 | 1.13 ± 0.09 A* | 0.9 ± 0.05 A**, B* | |
| 0.53 ± 0.16 | 0.66 ± 0.19 | 1.01 ± 0.27 | 1.72 ± 0.53 A***, C***, D* | 2.03 ± 0.43 A***, B***, E*** | |
| 0.07 ± 0.03 | 0.04 ± 0.03 | 0.03 ± 0.02 A* | 0.02 ± 0.01 A** | 0.02 ± 0.00 A** |
The levels of malodialdehyde (MDA) in kidney were higher in group 4 and 5 vs. control group (P = 0.001), group 2 vs. group 4 (P = 0.0001), group 3 vs. group 4 (P = 0.05), group 2 vs. group 5 (P = 0.001), group3 vs. group 5 (P = 0.001).
GSH of the kidney in experimental groups was significantly lower than the control in group 3, 4 and 5 (P = 0.03) for group 3 vs. C group and (P = 0.01) for group 4 and group 5 vs. C group.
5. Discussion
The current study shows that pathologic lesions in the kidney are responsible for treatment with sulfonamide. Histopohatlogical examination of the renal tissue revealed that sulfadiazine induces hydropic degeneration, tubular necrosis, glomerular and tubular atrophy, formation of hyaline cast, congestion, hemorrhage, interstitial nephritis and fibrosis. In the present study, dose-dependent sulfadiazine-associated nephrotoxicity has been identified. In the group 2 taken 2 mg sulfadiazine; changes were pretty mild and reversible. In group 3 with administration of 10 mg sulfadiazine, the changes were more severe though reversible yet. Group 4 and 5 received 30 mg and 70 mg sulfadiazine respectively. High dose administration caused both reversible and irreversible pathological changes with more severity compared to control and other treated groups. However, in group 5, irreversible injuries were more severe and increased fibrous tissue was noted.
Majeed et al. [9] studied the toxicological effect of sulfonamide in domestic pigeons by oral intubation of two dosage levels as intermediate 40 mg/kg and high 80 mg/kg. Histopathological results demonstrated nephrotoxic effects characterized by degenerate and/or dilated cortical tubules in intermediate while, high dose groups appeared glomerular atrophy some with dilated Bowman’s spaces, the severity of the changes were more intense in the high dose group, though the changes did not lead to necrosis.
In another study, Islam et al. [8] investigated the counteracting effect of Spirulina against potentiated sulfonamides side effects in rats. Resulting significant histopathological changes in the kidney of treated rats with sulfonamide (96 mg/rat/day) throughout the experimental period of 60 days, represent by of slight degenerative in the renal parenchyma. These results were compatible with the present study except we detected irreversible injuries like tubular necrosis in high dose administration (30 and 70 mg), and also, in our study, embryos received a single dose of sulfadiazine. Odigie [14] investigated the morphological alteration of visceral organs (kidney and liver) of albino Wister rats pre-exposed to prophylactic consumption of sulfonamide based drugs. Histological findings indicated that oral treatment by 3 and 4 mg drug for 21 days caused infiltration of inflammatory cells, congestion of glomerulus, hematomas, thickening of the interstitial cells and vacuolation with congestive and tubular necrosis as compared to the control group. However, in present cases, chicken embryos received 2 mg and 10 mg by in ovo injection, showed mild pathological changes.
Lebkowska-Wieruszewska and Kowalski [15] evaluated the residue depletion in healthy turkeys treated with sulfachloropyrazine. Results showed that sulfachloropyrazine has a long half-life and relatively high bioavailability. The drug was found in measurable edible tissues of turkeys eighteen days after the cessation of treatment.
Haritova et al. [3] studied pharmacokinetics of sulfachlorpyrazine-sodium in healthy chickens and chickens experimentally infected with Eimeria tenella. Results showed that pathological changes in E. tenella infected chickens contribute to slower absorption and elimination rate of sulfachlorpyrazine which resulted in higher accumulation of the drug in the body.
Malik et al. [5] studied effect of sulfonamides residues on egg quality traits resulting sulfonamide drug affected the external and internal quality of egg by decreasing egg shell weight, shell thickness, yolk height, yolk width and also yolk index. In this regard, it is suitable to escape eggs selling and consumption during treatment and withdrawal periods. The presence of sulfonamide residues necessitates the application of biosecurity measures at poultry farm level.
In oxidative stress, MDA content, has been increased by the higher concentration of sulfadiazine, on the other hand the lower concentrations were on the contrary. As shown in table 2 embryos that received sulfadiazine at dose 30 mg and 70 mg showed significant increase in MDA in the kidney tissue as compared to the control animals and group 2 mg and 10 mg sulfadiazine. These results were compatible by histopathological results.
GSH has been accepted as a ubiquitous sulfhydryl-containing molecule in cells that it is responsible for maintaining cellular oxidation-reduction homeostasis. Alterations in GSH homeostasis can be considered as an indication of functional-damage to the cells [16]. As shown in Table 2 sulfadiazine at dose 10 mg, 30 mg and 70 mg decreased GSH levels in the cells in a concentration dependent manner. Therefore, it can be assumed that the reduction in GSH concentration might cause the effectiveness of GST and GPx activity to be restricted, as evident by the intensification of lipid peroxidation [17]. We suspected that the observed increased concentration of lipid peroxides, along with decreased GSH was capable of inducing some injuries such as apoptosis visible at the cellular level. Concomitant cellular oxidative stress was revealed by reduced GSH levels, and amplified lipid peroxidation [16].
Oxidative stress (OS) plays a significant role in the pathogenesis of renal disease and its progression [18]. Kidney damage in OS-related Acute Kidney Injury (AKI) was associated with increased reactive oxygen species (ROS) production, leading to oxidation of several macromolecules (e.g. protein, DNA and lipid). Production of lipid peroxidation (LPO) in OS-related AKI results in large production of secondary products such as malondialdehye (MDA) and 4-hydroxynonenal [19, 20].
In conclusion, we have demonstrated that, dose-dependent administration of sulfadiazine significantly altered the histopathologic structure of renal tissues of chickens. Furthermore, the major histopathologic events in the course of sulfadiazine cytotoxicity are renal tubule epithelial cell necrosis, interstitial nephritis and fibrosis, formation of hyaline cast and congestion and hemorrhage. And also, sulfadiazine at dose 30 mg and 70 mg caused perturbation in antioxidant defense system by marked increase in LPO, and decrease in GSH.