1. Background
Kidney stone disease (or nephrolithiasis) is relatively common, with a lifetime risk of approximately 5% for both genders. A possible environmental cause as well as genetic predisposition is responsible for the increasing of kidney stone prevalence among both sexes of all ages [1]. During the last decades, a lot of studies have been done on the physical chemistry of stones formation. It is well known that crystallization processes alone cannot be able to explain the pathophysiology of kidney stone disease [2]. Five types of crystalline components in urinary tract stones have been reported: calcium oxalate, calcium phosphate, bacterial related, purine and cystine. A mixture of two or more of these components covers the majority of urinary stones. The most common component of kidney stones is calcium oxalate combined with apatite [3]. One of the most frequent infections in developed countries is UTIs (Urinary tract infections). uropathogenic Escherichia coli (E. coli), a non-urea-splitting bacterium, represent 80% of uncomplicated UTIs. Increasing of the fitness of bacteria causing UTIs during infection is highly associated with their virulence factors [4]. Some of E. coli virulence factors are adhesins (Type 1 pili, P fimbriae, Dr-family pili, S fimbriae, F1C fimbriae) and toxins Cytotoxic Necrotizing Factor-1 and Secreted autotransporter toxin (CNF1 and Sat) [5]. FimH is a member of E. coli fimbriae family, which participate in bacterial adhesion [6] and has a role in diseases related to bacterial autoaggregation such as UTI [7, 8].
Urinary tract infections are associated with kidney stone formation [9]. Furthermore, antimicrobial resistance has been frequently reported in bacteria separated from stone formers with UTIs [10].
Perviously, several studies have shown that the most prevalence of bacteria associated with UTI is E. coli [11], and oxalate calcium is the most frequent component of kidney stones [11]. Also some studies have indicated the relation of FimH gene and UTI [12]. Although the association between kidney stone disease and UTIs is frequently observed, more studies should be done on its prevalence, antimicrobial susceptibility pattern and its causative microorganisms [13]. Also there is rare study on the association between FimH and kidney stone formation in our country.
2. Objectives
Therefore, the purpose of this research was to study on stones and identify the bacteria in patients’ kidney stones attending to Hashemi-Nejad hospital (Tehran, Iran). We also measure the frequency of fimH gene and its related protein in E. coli isolated from the patients to find out the effect of this protein in kidney stone formation.
3.Patients and Methods
3.1. Samples
In this observational-descriptive study, 40 kidney stone samples were gathered from Shahid Hashemi-Nejad hospital by PCNL (percutaneous nephrolithotomy) method. A piece of each sample was used for culturing of kidney stone bacteria and the remained amount was applied for further analysis. All patients gave written informed consent.
3.2. Kidney Stone Analysis
The composition of each sample was determined using a kidney stone determination kit (IranKav, Iran). The kit contains all necessary reagents for semi-quantitative measurement of the most important components of kidney stones including calcium, oxalate, magnesium, phosphate, ammonium, uric acid and cystine. Initially a homogenous powder was prepared from each sample and dissolved by adding some pure sulfuric acid and the final volume was adjusted to 50 mL using distilled water. The prepared samples were used for determination of different parameters using the mentioned kit.
3.3. Kidney Stones Culture
In order to analysis the bacterial component of each sample, according to Hugosson method a piece of each stone was added to BHI (Brain heart infusion) broth and another piece was added to Thioglycolate medium. The medium was incubated for 48 h at 37ºC and then applied for presence of microorganisms. Different culture media such as Baird parker, DNase, MSA (Manitol salt agar), etc. were applied for discrimination of different kinds of bacteria.
3.4. Determination of Fimh Gene Frequency in E. coli Separated From Kidney Stones
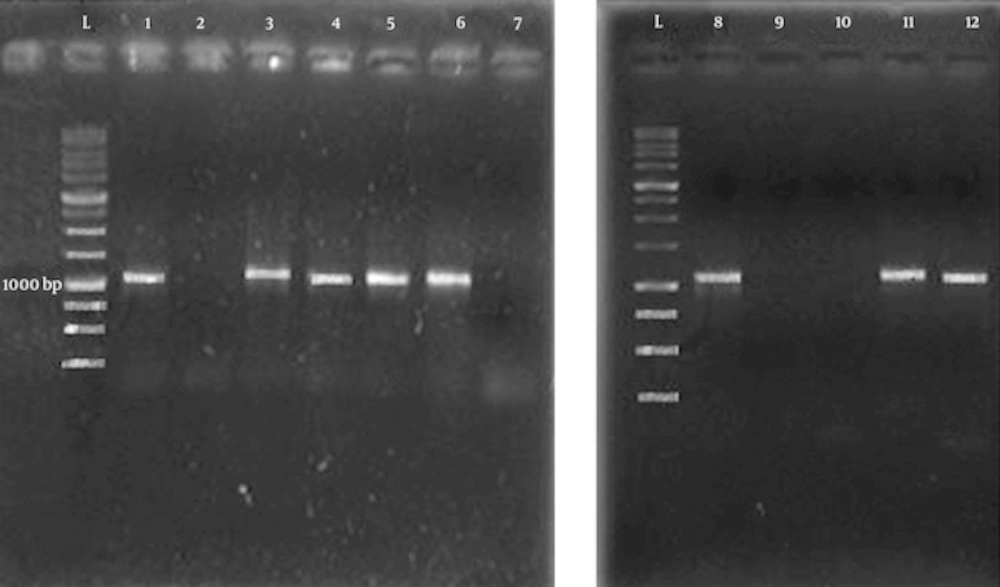
Since the number of separated E. coli bacteria from previous steps was small (found in five samples), 42 further samples were gathered from UTI patients and seven samples were diagnosed to have kidney stones containing E. coli. Genomic DNA of E. coli bacteria of these 12 samples was extracted using MBST kit (Roshd, Iran). The fimH gene was amplified by PCR using following primers: Forward (5'-TCCCGTTACAGGTCAGAGC-3'), Reverse (5'-GTGCAGGTTTTAGCTTCAGG-3') [12]. The PCR program was as follow: (pre-denaturation at 95ºC for 5 min, followed by 25 cycles at 95ºC for 1 min, 55ºC for 30 seconds, 72ºC for 1 min and a final-extension at 72ºC for 5 min). The PCR product was run on a 1% agarose gel and the bands were visualized using transilluminator.
3.5. Fimh Protein Extraction From E. coli Bacteria
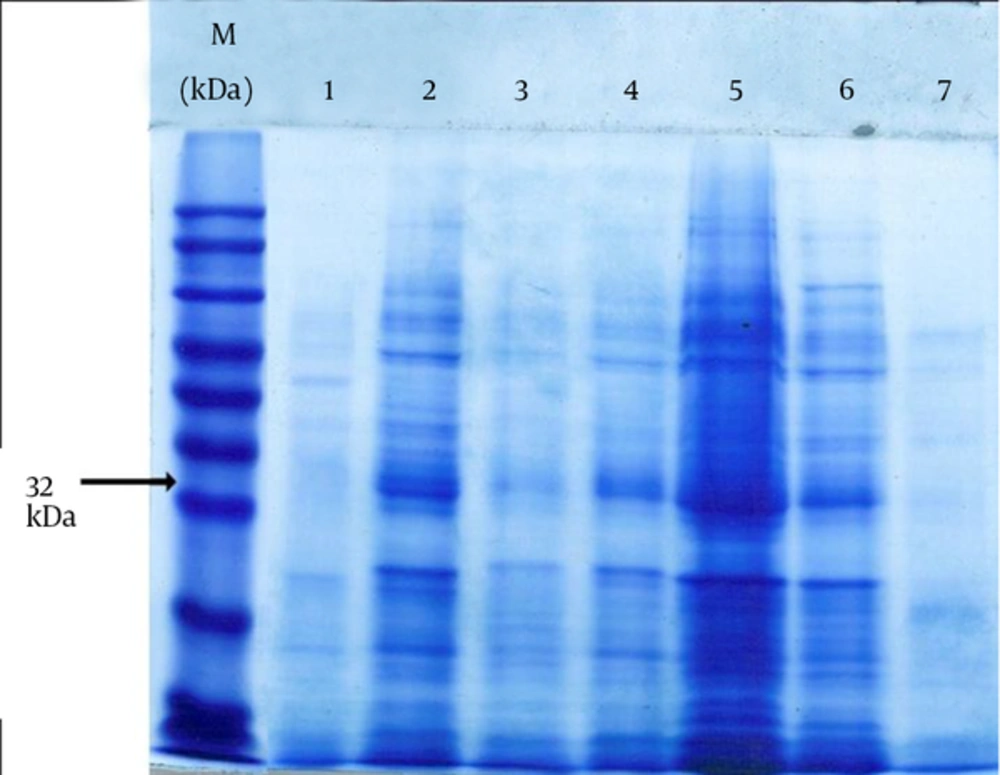
In order to extract FimH protein, TES [Tris-HCl, ethylenediaminetetraacetic acid (EDTA) and sucrose] solution (Sigma, US) was used and some distilled water was added gradually to find the optimum condition in which only the periplasmic proteins are released without cell lysis. Initially 1.5 mL of bacterial culture was centrifuged at 4ºC 5000 g for 5 min. The supernatant was removed completely and the pellet was dissolved at TES solution and placed on ice and slightly shook for 20 min. After adding 1.5 mL distilled water the shaking on ice was continued to 35 min more. After osmotic shock, in order to isolate periplasmic proteins, cell samples were centrifuged at 4ºC, 14000 g for 20 min. The supernatant contained periplasmic proteins and the pellet contained spheroplastic proteins. Extracted proteins were confirmed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
3.6. Statistical Analysis
Data were analyzed using Graphpad prism 6.01 and also SPSS-16 programs. Data were compared with each other by student t-test. P values ≤ 0.05 were considered significant.
4. Results
4.1. Frequency of Kidney Stones:
From 40 kidney stones samples, 12 samples (30%) separated from women and 28 samples (70%) belonged to men. According to the hospital documents, 55% of studied samples were from tropical regions (south and north of Iran), 30% were from moderate regions (the center of Iran) and the remained ones were from other places.
4.2. Kidney Stone Composition and Bacteria
In order to define the structure and identity of separated kidney stone, the samples composition and bacterial content were obtained. Table 1 summarizes the composition of kidney stones samples. As it shows, the most prevalence of stones belongs to calcium oxalate (whewillite) stones. The most frequent bacterium in kidney stones was E. coli (29.41%). The percent of different kinds of bacteria in the samples is summarized in table 2.
In the present study, no microorganism was observed in 23 of 40 kidney stones culture. The frequency of isolated microorganisms in kidney stones cultures was as follow: E. coli (29.41%), Staphylococcus spp. (17.64%), Klebsiella spp. (11.76%) and Proteus spp. (5.8%). Although E. coli produces a little amount of urease, it was seen in 29.41% of the patients, raising a question whether urease-producer bacteria are necessary for kidney stones formation or not. To answer to this question, it could be proposed that we might be unable to recognize some microorganism such as mycoplasma (Ureaplasma urealyticum, Bacteroides, etc.) with common experimental methods. Another possibility is that urease-producer microorganisms initially appear as transient infection and disappeared after kidney stone formation. It is also possible that kidney stones can be formed in presence of non-urease-producer bacteria.
| Kidney stone type | Prevalence (%) |
|---|---|
| Whewillite | 63.40 |
| Brushite | 15.07 |
| Apatite | 3.62 |
| Struvite | 1.25 |
| Uric acid | 9.57 |
| Cystine | 0.75 |
| Bacteria | Prevalence (%) |
|---|---|
| E. coli | 29.41 |
| Staphylococcus | 17.64 |
| Klebsiella | 11.76 |
| Entrobacter | 5.88 |
| Citrobacter | 5.88 |
| Proteus | 5.88 |
| Ervinia | 5.88 |
| Bacillus | 5.88 |
| Klebsiella-Bacillus | 11.76 |
| Not-detected | 0.03 |
4.3. Frequency Of Fimh Gene And Fimh Protein
From 12 E. coli samples, eight samples have fimH gene which was amplified by PCR and four samples were negative for this gene. FimH protein was obtained from six samples (a ~32 kDa band on SDS-PAGE) and other six samples did not have the FimH protein band (the SDS-PAGE result of some samples is shown in Figure 2).
5. Discussion
From 40 studied kidney stones samples, 28 samples (70%) were separated from male patients and 12 samples (30%) were separated from females. It shows that male patients are significantly more prone to kidney stones formation in comparison with females. The main calcium stone was calcium oxalate stone with a frequency of 63.4%. The frequency os struvite stone was 1.25%. The lowest prevalence of stone was cystine. All samples were 82 (40 samples of kidney stones and 42 samples of UTI). E. coli was found in 12 samples of all patients. The frequency of fimH gene in isolated E. coli was 57.14% by PCR. Protein extraction was from separated E. coli bacteria. The FimH protein was seen only in 50% of our samples by SDS-PAGE.
It is noteworthy that similar to our study, Stamatelou et al. reported a prevalence of 6% and 12% of kidney stones for female and male subjects, respectively [14]. Rivera declared that the most prevalence of kidney stone is seen where the temperature and moisture are high [15]. Similarly, in the present study, 30% of studied subjects were from tropical regions (south and north of Iran), 30% were from moderate regions (the center of Iran), and the remained ones were from other places. These results suggest that the formation of kidney stone may increase in tropical regions. Kramer et al. declared that calcium oxalate stones formed by E. coli cover the most frequent quota of urinary stones in the western world. They showed that the urinary stones may be initially formed and then be infected with bacteria. They also announced that the bacteria which are the main reason of chronic urinary tract infections and exist inside the urinary tract (urea-splitting bacteria) may cause stone formation. Although the origin of struvite stones may be de novo, often urea-splitting bacteria infect pre-existing stones. There is evidence showing that UTI caused by urea-splitting bacteria are not exclusively correlated with the struvite stones formation [16]. The frequency of struvite stone was 1.25% in our study. Ansari et al. studied on 1050 kidney stone samples. In their study, the prevalence of calcium oxalate, struvite, apatite and uric acid stones were 93.04%, 1.92%, 1.48%, and 0.95% respectively and 2.96% of samples were consisted of a mixture of different stones [17]. In Akagashi et al. study, struvite and calcium oxalate stones covered 32.1% and 22.2% of kidney stones samples [18]. The frequency of struvite stones was determined 15 - 20% in Griffith study [19]. Gomez-Nunez et al. showed a frequency of 2% for struvite stones and declared that nowadays the frequency of this stone is decreasing with unknown reasons [11]. This information demonstrates a large difference of struvite stones prevalence. As Gomez-Nunez et al. suggested, this inconsistency may refer to differences in ethics, genetics, geographic location, nutrition, lifestyle and metabolism [11]. In our study uric acid stones covered 9.75% of kidney stones. In a study by Parmar, 5 - 10% of studied kidney stones were uric acid stones [20]. The prevalence of uric acid stone was reported 10% in Coe et al. study [21]. Our results are consistent with Parmar and Coe et al. results. Tavichakorntrakool et al. obtained a prevalence of 14% for uric acid stones which is inconsistent with our study [22]. This inconsistency may refer to nutrition, climate, job, etc. In the present study, calcium stones covered 82.09% of all stones. The main calcium stone was calcium oxalate stone with a frequency of 63.4%. A frequency of 75 - 80% was determined for calcium stones in Pak study and the main calcium stone of their study was calcium oxalate [23]. Similarly, Coe et al. report a prevalence of 80% for calcium stones with predominant frequency of calcium oxalate stones [21]. Calcium stone prevalence was determined 77% in Tavichakorntrakool et al. study, which among them calcium oxalate covered 51% of cases [22].
Abrahams and Stoller showed that 67% of calcium oxalate stones contain phosphate in their center [24]. However this frequency was reported 90% in Grases et al. study [25]. Consistent to mentioned researches, our results also showed a high prevalence for calcium stones with a predominant frequency for calcium oxalate stones. Also 18.92% of our calcium stones were pure calcium oxalate and in 63.7% of calcium stones phosphate was observed. These results were also consistent with previous studies. The prevalence of cystine stones was determined 0.75% in our study. This prevalence was reported 1.4% by Ciftcioglu et al. [26]. Although our result is not consistent with Ciftcioglu et al. study, as declared by Parks and Pearle, the cystine stones are scarce and they are not clinically important [27]. In this study, 40 kidney stones in addition to 42 UTI patients were studied. Stones were also found in seven patients of UTI subjects. E. coli was found in 12 samples of all patients.
The frequency of fimH gene in isolated E. coli was 57.14%. Usein et al. studied on 78 E. coli isolated from urinary tract infection [28]. The frequency of fimH gene in their study was 86%. This frequency was determined 83% in Kaczmarek et al. study [29]. Khorshidi et al. studied on 313 child fecal samples and reported the prevalence of 98% for fimH gene [30]. In other researches by Karimian et al. on 123 E. coli samples [31] and Moreno et al. on 21 E. coli samples [32] this frequency was reported 79.67% and 95% respectively.
In contrast with mentioned studies, the fimH gene frequency in Lichodziejewska et al. [33], Pere et al. [34], and Kisielius et al. [35] studies were 38%, 45% and 76% respectively. This large discrepancy in previous studies may refer to the different environments because it has been shown that the fimH gene prevalence is affected by environmental factors [36]. Furthermore this discrepancy may be occurred because of the phase changes of bacteria as a direct correlation exists between the fimH gene frequency and subculturing of bacteria. In the initial studies on fimH, the mannose-sensitive hemaglutinin function was only observed in two of 24 urine samples but it increased up to 11 samples after subculturing of the samples [37]. These studies suggest that fimH gene frequency strictly depends on the study conditions. Although FimH protein was seen only in 50% of our samples (and it could be due to weakness of the extraction process), it seems that fimH gene may have a role in kidney stone formation or progression because a significant correlation was seen between fimH gene and the formation of kidney stone. It should be noted that the kidney stones formation is not necessarily dependent on fimH gene and other factors such as pap, cnf-1, etc. may play a role in kidney stones formation. Our data indicated that kidney stone disease and UTIs are not only associated with struvite stones and almost all chemical types of kidney stones may involve in UTI and kidney stone formation. We also realized that although E. coli is a non-urea splitting bacteria, it is the most causative microorganism found in urine and stone. Finally we recognized that fimH gene is seen in the majority of kidney stone samples so it may have a role in forming of kidney stone, although it should be more clarified in future studies.