1. Background
Cerebrovascular stroke is one of the most frequent reasons of disability in developed countries. According to the WHO report in the year 2004 it causes significant physical, emotional and cognitive disabilities among survivors, accounting for 3.6% of the total disability-adjusted life years [1-3]. Few patients will recover completely (10%) and some of them die shortly after the stroke (15%), therefore a lot of patients recover with any impairment or need to home nursing [1, 2]. Recently, the increasing amount of remaining impairments and disabilities has focused concern in interpolations that might precipitate rehabilitation [3, 4]. In the acute phase of recovery, post-stroke depression can impress functional recovery of physical therapy outcome in short-term and long-term and initiation of antidepressant drugs as soon as possible after stroke may preclude the appearance of post-stroke depression [1-3]. Selective serotonin reuptake inhibitors (SSRIs) have been prescribed for many years to treat post-stroke depression [1, 2]. Good functional recovery has been reported with prescription of antidepressant drugs in some studies [1-3, 5-7]. Furthermore SSRIs may be effective in post-stroke emotional incontinence [3, 4]. The few small clinical trials of SSRIs all suggest that these drugs have a positive effect in motor improvement after stroke [1, 6]. In one study the effect of fluoxetine for motor recovery of patients after ischemic stroke has been assessed and they found that early prescription of fluoxetine and physiotherapy enhanced motor function in these patients [5]. They used Fugl-Meyer motor scale as an assessment which focuses on motor improvement. There are various mechanisms for describing the physiology of post-stroke depression and one of them is decreased monoamine synthesis due to enzyme inhibition during ischemia, and decrease in norepinephrine and serotonin construction from ascending axonal projections to the cortex [7-9]. Drugs that act as selective serotonin reuptake inhibitors (SSRIs) have frequently been used in managing these patients, almost always to conflict the symptoms of depression, which are common in these patients and compromise their progression. Treatment alone is not always enough to combat post stroke complications.
2. Objectives
In this trial, we assessed the effect of 90 days treatment with fluoxetine in motor improvement of post-stroke patients and compared it with placebo.
3. Methods
In this double-blind trial study, allocation of patients in two groups was done with random permuted blocks. Random permuted blocks are blocks of different sizes, where the size of the next block is randomly chosen from the available block size. The follow up duration for patients were 90 days. Participants were patients who suffered an acute ischemic stroke that were being documented with radiologic imaging in territory of middle cerebral artery (MCA). The study protocol was approved by the ethics committee of Kermanshah University of Medical Sciences and registered in IRCT (Iran research clinical trial) with serial number 201312088323N7.
Informed consent was obtained from each patient (or his/her attendants) before entry into the study. The inclusion criteria were as follows: acute ischemic stroke in the territory of MCA (documented with a CT scan) that leads monoparesis, hemiparesis or hemiplegia. The patient did not in comatose state and was stable. The exclusion criteria were death due to any cause during the assessment, pregnancy, poor compliance of drugs and physiotherapy, miscarriage returning of patient for further exams and assessments, any drug complication during assessment (prospected or not), any metabolic disease (liver, renal, cardiac impairment and hyperthyroidism), ischemic stroke in the territory of anterior cerebral artery (ACA) or posterior cerebral artery (PCA), using of any interfering drugs with fluoxetine (such as: cyproheptadine, selegiline and.
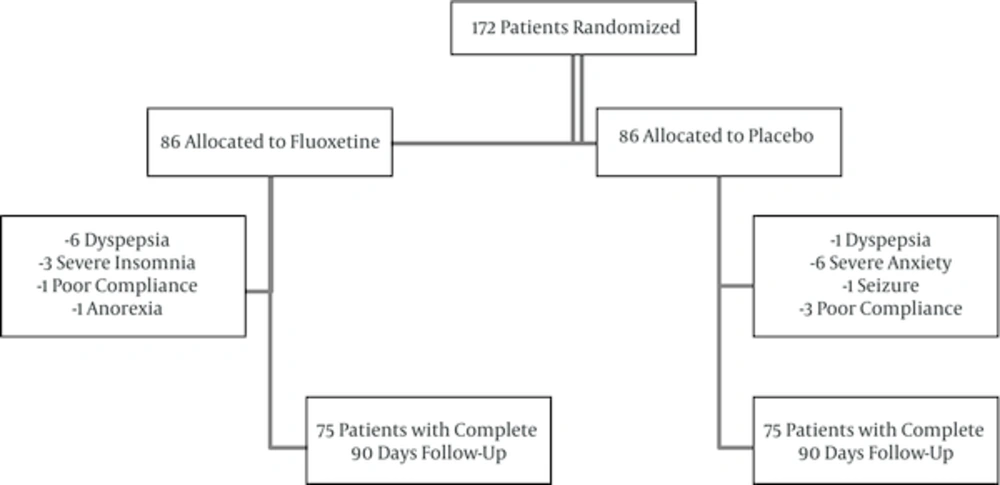
Between 22 June 2013 till 7 September 2014, 172 patients who had an acute ischemic stroke and admitted in Imam Reza and Farabi hospitals (two neurology centers in Kermanshah, Iran) according to random permuted blocks were randomly assigned into two groups: the fluoxetine group (n = 86), which included patients treated with fluoxetine (20 mg, PO1, daily); and the placebo control group (n = 86), which included patients treated with placebo that was identical to the active drug in appearance and packaging. Fluoxetine and placebo capsules were both prepared by the Iranian EXIR pharmaceutical company. Every patient was examined within the first days of stroke (during admission) and at the time of discharge, 45 days and 90 days after stroke in outpatient clinic. The examinations included neurological examination, Barthel Index (BI) and Hamilton depression rating scale (HDRS). The patients were visited in 45 and 90 days after stroke (in the hospital) for the aim of undertaking a BI and HDRS. All patients had 30 sessions of routine physiotherapy during the rehabilitation period. Baseline demographics and a detailed medical history of all participants were recorded.
The BI is an ordinal scale used for assessment of disability in daily activities and yields a score of 0 - 20. A higher number is associated with a greater likelihood of independence. Its reliability is 0.90 (95% CI). Symptoms of depression and anxiety were assessed through the HDRS (the Chronbach α was 0.797) (95% CI) and validity was 0.822 (P < 0.001). The questionnaire is designed for adults and is used to rate the severity of their depression by probing mood, feelings of guilt, suicide ideation, insomnia, agitation or retardation, anxiety, weight loss and somatic symptoms. Each item on the questionnaire is scored on a 3 or 5 point scale, depending on the item and the total score is compared to the corresponding descriptor. Every changing in level of HDRS (0 - 7 = normal level, 8 - 13 = mild level, 14 - 18 = moderate level, 19 - 22 = severe level and > 23 very severe level) and 2 point increments in BI was considered good prognosis. For example improvement in BI from score 11 to 13 or HDRS from score 16 (moderate depression) to score 11 (mild depression) considered a good prognosis. Brain imaging (CT scan), as well as hematologic, thyroid function and biochemical laboratory tests were available for all participants.
3.1. Statistics
Assessments of patients were done on day 0 (baseline) and then 45 days and 90 days after registration with BI and HDRS scales. According to Kolmgrov-Smearnov test, data of BI and HDRS in days 0, 45 and 90 had not normal distribution in both fluoxetine and placebo groups (P < 0.05).
The data was analyzed by Mann-Whitney U test, Wilcoxon Test, χ2 test and Cluster analysis). Statistical significance was considered at P ≤ 0.05. Statistical analyses were performed using SPSS-16 software. The Wilcoxon test was used to determine the statistical significance of the differences in the BI and HDRS scores between the three evaluations in each treatment groups separately. The Mann-Whitney U test was used to evaluate the statistical significance of the differences in outcome measures (BI and HDRS scores) among the two treatment groups at entry, middle and at the end of the study. We used cluster analysis to separate patients into two groups with poor or good outcome by means of improvements in both BI and HDRS scores from study initiation to day 90. The χ2 test was used to evaluate the difference in the number of placebo and fluoxetine-treated patients allocated to the poor or good recovery groups. Descriptive statistics calculated for these data were median and quartiles.
4. Results
As shown in Figure 1, of 172 patients who met the criteria, 22 patients were excluded. Therefore 150 patients were enrolled in the full-set analysis. Baseline and demographic characteristics and risk factors of patients are in table 1. The main adverse events were dyspepsia, anxiety, severe insomnia, seizure, and anorexia (Table 2). In none of the previous such studies, there is no addressing to effect of risk factors on BI and HDRS scales. However, as shown in Table 1, patient’s demographic characteristics and risk factors had not significant statistical differences. Therefore, they had not confounding effect in our study. One of adverse events in placebo group was serious (seizure), but treatment was not interrupted in patients with these adverse events. In both patient’s demographic characteristics and risk factors there were no significant statistical differences. As shown in Table 3 according to Wilcoxon test, in each fluoxetine and placebo groups, there were significant statistical differences in BI scale in day 0 with 45, in day 45 with 90 and in day 0 with 90 (P = 0.001). Also in forenamed test for HDRS scale, there were significant statistical differences in fluoxetine group in day 0 with 45, in day 45 with 90, and in day 0 with 90 (P = 0.001). On the other hand, HDRS scores in the placebo group in day 0 with 45, in day 45 with 90, and in day, 0 to 90 were not significantly different. According to Mann-Whitney U test, changes in BI and HDRS between fluoxetine and placebo groups between days 0 to 45, 45 to 90, and 0 to 90 was statistically significant (P = 0.001) (Table 4).
| Characteristics | Fluoxetine | Placebo | P Value |
|---|---|---|---|
| Age | 63.2 ± 11.4 | 64.6 ± 11.9 | 0.21 |
| Sex, No. (%) | |||
| Male | 38 (50.6) | 31 (41.3) | 0.39 |
| Female | 37(49.3) | 44 (58.6) | 0.25 |
| Risk Factors,No. (%) | |||
| Diabetes | 23 (30.6) | 18 (24) | 0.35 |
| Hypertension | 42 (56) | 38 (50.6) | 0.51 |
| Smoking | 31 (41.3) | 42 (56) | 0.07 |
| Cardiac disease | 11 (14.6) | 18 (24) | 0.14 |
| Previous stroke | 13 (17.3) | 16 (21.3) | 0.53 |
Patients’ Demographic Characteristics and Risk Factors
| Fluoxetine (n = 75) | Placebo (n = 75) | P Value | |
|---|---|---|---|
| Dyspepsia | 6 (8) | 1 (1) | 0.11 |
| Anxiety | 0 | 6 (8) | 0.13 |
| Severe insomnia | 3 (4) | 0 | 0.24 |
| Seizure | 0 | 1 (1) | 0.99 |
| Anorexia | 1 (1) | 0 | 0.99 |
Frequency of Adverse Events (Percent) in Follow-Up Duration
| BI Before Therapy | BI Day 45 | BI Day 90 | HDRS Before Therapy | HDRS Day 45 | HDRS Day 90 | |||
|---|---|---|---|---|---|---|---|---|
| Fluoxetine Group | Mean ± SD | 12.59 ± 2.60 | 13.97 ± 2.52 | 15.68 ± 2.61 | 9.80 ± 3.47 | 9.14 ± 3.18 | 8.14 ± 2.90 | |
| Percentiles | 25 | 11.00 | 13.00 | 14.25 | 8.00 | 7.00 | 6.00 | |
| Median | 13.00 | 14.00 | 16.00 | 9.00 | 9.00 | 8.00 | ||
| 75 | 14.00 | 16.00 | 17.00 | 11.00 | 11.00 | 10.00 | ||
| Placebo Group | Mean ± SD | 12.56 ± 1.56 | 13.43 ± 1.57 | 14.26 ± 1.80 | 8.40 ± 2.33 | 8.48 ± 2.22 | 8.25 ± 2.28 | |
| Percentiles | 25 | 11.00 | 12.50 | 13.00 | 6.00 | 7.00 | 7.00 | |
| Median | 13.00 | 13.00 | 14.00 | 8.00 | 8.00 | 8.00 | ||
| 75 | 13.00 | 14.00 | 15.50 | 10.00 | 9.50 | 9.00 |
Percentiles and Mean Scores of BI and HDRS Before Treatment and Days 45, and 90
| BI 0 - 45 | BI 45 - 90 | BI 0 - 90 | HDRS 0 - 45 | HDRS 45 - 90 | DRS 0 - 90 | |||
|---|---|---|---|---|---|---|---|---|
| Fluoxetine group | Mean ± SD | 1.38 ± 1.06 | 1.71 ± 1.34 | 3.09 ± 1.57 | 0.66 ± 1.38 | 1.00 ± 1.38 | 1.66 ± 1.68 | |
| Percentiles | 25 | 2.00 | 3.00 | 4.00 | 0.00 | 0.00 | 1.00 | |
| Median | 1.00 | 2.00 | 3.00 | 0.00 | 1.00 | 1.00 | ||
| 75 | 1.00 | 1.00 | 2.00 | 1.00 | 2.00 | 2.00 | ||
| Placebo group | Mean ± SD | 0.87 ± 1.13 | 0.82 ± 0.95 | 1.70 ± 1.33 | 0.08 ± 1.30 | 0.23 ± 1.23 | 0.14 ± 1.65 | |
| Percentiles | 25 | 1.00 | 1.00 | 2.00 | 1.00 | 0.00 | 1.00 | |
| Median | 1.00 | 1.00 | 1.00 | 0.00 | 0.00 | 0.00 | ||
| 75 | 0.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| P-Value | 0.001 | < 0.001 | < 0.001 | 0.001 | 0.004 | < 0.001 |
Changes in BI and HDRS Between Days 0 to 45, 45 to 90, and 0 to 90 in Both Fluoxetine and Placebo Groups
| Prognosis of Groups | Good | Poor | Total |
|---|---|---|---|
| Fluoxetine | 55 (73.3) | 20 (26.7) | 75 (100) |
| Placebo | 17(22.7) | 58(77.35) | 75(100) |
Prognosis of Patients After Rehabilitation Therapy (Percent)
According to the χ2 test, there was a relation between treatment with fluoxetine and functional improvement in comparison with placebo (OR = 2.9, P = 0.004) and Improvement in BI from study initiation to day 90 in fluoxetine-treated group is significantly higher in comparison with placebo-treated group (OR = 8.6, P = 0.001). The cluster analysis, as already explained in methods and materials session, based on improvement in both HDRS and BI scores was used to separate patients into two groups: the level of functional improvement in the poor prognosis group in BI was 1.9 ± 0.91 and in HDRS was 0.05 ± 1.31. The level of functional improvement in good prognosis group in BI was 3.5 ± 1.51 and in HDRS was 2.25 ± 1.54. Good prognosis group comprised 55 patients (73.3%) treated with fluoxetine and 17 patients (22.7%) treated with placebo (Table 5).
5. Discussion
Based on the results of our study, 90 days treatment with fluoxetine is more effective than placebo in disability and depression in patients with acute ischemic stroke. Furthermore, fluoxetine treatment yielded a significantly larger number of patients with good outcome compared to placebo. In comparison to the other similar studies, larger sample size was selected [10].
This study emphasizes the need for early recognition and treatment of functional impairment after stroke. On the other hand, nearly all stroke patients have other metabolic disorders such as hypertension and diabetes mellitus, and fluoxetine has no interaction with these illnesses and their treatments, and this is another advantage of this drug. It is known that the depression that may happen to these patients after they have tortured a stroke is not only a reaction to the patient’s situation caused by the complication, but is also an organic condition caused by impairment of cerebral functions and pathways that have been injured by the stroke [11, 12]. If a patient with stroke is left untreated and unmanaged, it can worsen a number of other common post-stroke conditions such as malnutrition, incontinence, pain, fatigue and sleep problems. Long-term fluoxetine treatment increases serotonergic transmission by blocking 5-HT reuptake locations or by upregulating 5-HT (5-hydroxy tryptophan) release that motivates motor function and possibly the recuperative routes that follow a brain injury [7]. Controlled studies on the effectiveness of fluoxetine in the rehabilitation of acute ischemic stroke are few and all of them include a small number of patients.
A previous study by Chollet et al. in their study surveyed the effect of fluoxetine in comparison with placebo in motor improvement of post-stroke patients [5]. They founded that early treatment of post-stroke patients with fluoxetine enhanced motor recovery after 3 months. Another study by Dam et al. showed that fluoxetine in comparison with maprotiline and placebo influenced functional outcome after ischemic stroke [13]. They allocated 52 patients (18 with fluoxetine, 17 with maprotiline and 17 with placebo). At the end of their study, they concluded that fluoxetine influenced the functional outcome after ischemic stroke. However, they offered more investigations of serotonergic drugs in stroke survivors. Pariente et al. in a double-blind placebo-controlled study showed that prescription of fluoxetine improved motor function of hand with a positive effect on motor cortex on functional MRI [14]. Zittel et al. showed the effect of citalopram in chronic post-stroke patients in a double-blind, placebo-controlled trial [12]. In conclusion, this study describes that fluoxetine may inversely encourage functional outcome after ischemic brain injury in stroke survivors.
Although the results of our study that show the efficacy of fluoxetine on motor improvement of patients after ischemic stroke, we had potential limitation in this study that was measurement errors caused by the effect of cognitive impairment and cooperation of patients. Therefore, we requested from patients’ attendants to evaluate problems. Despite of our larger sample size, we recommend a larger sample size and longer duration prospective future studies of this field would provide stronger evidence for effectiveness of serotonergic drugs, especially fluoxetine in rehabilitation of motor deficits after ischemic stroke.