1. Background
Today the fertility return is considered as the major problem after treatment for children with cancer because chemotherapy or radiation therapy in cancer patients has the risk of infertility. For children before puberty (or perhaps some men), freezing the immature testis tissue before treatment and then returning it to the body of the person as soon as termination of the treatment is one of the methods that may permanently can return the normal fertility to the patient. In animal models (rodents, pigs, goats and dogs), transplantation of freezing testicular spermatogonial stem cell into the testes of infertile male can lead to restore spermatogenesis [1]. With this operation it is possible to combat the extinction of endangered species, in addition; we can strengthen the power of reproduction of the species. Despite of many advances in technique innovation and preparing freezing solutions that have occurred since the first freezing of testis in 1960s to recent years, still these techniques do not show good result [2]. A number of researchers know the specific biological characteristics of the testis and many issues that spermatogonial cells are faced with during bearing the freezing methods, as the main reason for it. Testis tissue undergoes important changes, such as ice formation, changing the internal structures of the cell, volume changes, functional changes and destruction of the membranes [3].
One of the most important and common injuries during freezing-melting process is oxidative stress that severely affects on testis tissue. Sperm cells (and spermatogonia) are sensitive towards oxygen that released from lipid peroxidation, thus it causes to membrane destruction and reduction of sperm motility and fertility [4, 5]. Uncontrolled production of ROS (reactive oxygen species) by immature sperms and leukocytes in seminal fluid may lead to disruption of natural sperms. All cellular components are exposed to ROS attack. ROS can cause severe pathophysiological effect on cell [6]. In confirmation, our findings have shown that increasing of ROS and decreasing of GSH (glutathione) can cause to increase lipid peroxidation that leads to vacuole induces, seminiferous tubes atrophy, apoptosis of generative cells [7, 8]. Optimization of freezing-melting environment may reduce the fatal side effects of the freezing solutions and processes to a minimum amount. Research results show that protecting the sperm plasma membrane against oxidative reactions during freezing protection is carried out by antioxidants. In fact, antioxidants change the cellular conditions by reduction of free radical oxygen production so that the cells and tissues have the least damage [4, 5, 9].
Various studies have been done on the effect of antioxidants on cellular parameters during freezing. For example, Breininger et al. showed that alpha-tocopherol prevents from oxidative reactions due to freezing protection of sperm [10]. Vitamin E (Vit E) is known as the most important fat-soluble antioxidant. Its main function is to protect the fatty acid chain of the cell membrane. Lipid peroxidation of this layer disturbs the function and permeability of membrane and consequently the cell will destroy and die. Cerolini et al. proved that adding vitamin E will prevent oxidative reaction effects of pig sperm during freezing [11]. Vitamin C (Vit C) is a water soluble antioxidant that addition of scavenger of free radicals, resulting in the re-entry of other antioxidants such as vitamin E and urate into the circulation, thereby reducing the oxidation of extracellular. Studies show that daily consumption of a certain amount of this vitamin, it protects DNA from degradation and oxidation [12].
2. Objectives
Since a wide variety of studies have been confirmed the antioxidant and protective effects of vitamin E and C, this study has sought to study antioxidant effects of these two vitamins separately and in combination on immature testicular tissue (seminiferous tubes) in two freezing-thawing processes (fast freezing-thawing and slow freezing-thawing) compare with control group and each other.
3. Materials and Methods
In this experimental study, all experiments were performed in accordance with principles of laboratory animal care. Male 6-day old BALB/c mouse pups (n = 16) were obtained from Razi herbal medicines research center. Mice were euthanized by excessive doses of ketamine HCl (80 mg/kg) and xylazine (10 mg/kg) (Pharmacia and Upiohn, Erlangen, Germany) [13] in accordance with the protocols approved by the Lorestan University Medical Science animal care and use committee. Every effort was made to minimize the number of animals used and their suffering.
Sixteen immature male mice (6 - 8 day old) were randomly selected, and the testes were removed surgically. Thirty two testes were divided randomly into 4 groups; control group and three experimental groups including vitamin E, vitamins C and vitamins E and C.Preparing the freezing solutions: First for each of the groups, 4 freezing solution were prepared in the basic culture 1, 2, 3 and 4, respectively (the basic cultures details are given in the Table 1) [1]. These solutions include four solutions of 0.1 mM vitamin E, four solutions of 1 mM vitamin C, four solutions of vitamins E and C with mentioned doses [14] and four control(Ctrl) solution that contains only the basic culture in the Table 1.
| Thawing Solutions | DMEM | DMSO | Ethylene Glycol | FBS | 0.5 Molar Sucrose |
|---|---|---|---|---|---|
| Solution 1 | 85 | 75 | 75 | - | + |
| Solution 2 | 70 | 15 | 15 | - | + |
| Solution 3 | 50 | 15 | 15 | 20 | + |
| Solution 4 | 50 | 15 | 15 | 20 | + |
Abbreviation: DMSO, Dimethyl sulfoxide.
aValues are expressed as %.
3.1. Fast and Slow Freezing Procedure
For each group, after removing the testes of mice body, testicular tunica albuginea were perforated with an insulin needle. Then the testes were transferred to its proprietary solution 1, 2 and 3, respectively (any solution for 10 minutes). In order to entering testes to fast freezing-thawing procedure, 4 testes removed from solution 3 and were transferred to half of the solution 4 and were transferred to liquid nitrogen tank. in order to entering testes to slow freezing-thawing procedure, 4 of the remaining testes were transferred to the other half of the solution 4 and were maintained at 4°C for one hour, at -20°C for one hour, at -70°C for 24 hours, respectively. Finally, were transferred to liquid nitrogen tank [15].
3.2. Preparing the Thawing Solutions
Before removing the testes from nitrogen tank, for each of the groups, 4 thawing solution were prepared in the basic culture 1, 2, 3 and 4, respectively, (the basic cultures details are given in the Table 2) [1].
| Thawing Solutions | DMEM (%) | FBS (%) | Sucrose (Molar) |
|---|---|---|---|
| Solution 1 | 100 | - | 0.5 |
| Solution 2 | 100 | - | 0.25 |
| Solution 3 | 100 | - | 0.125 |
| Solution 4 | 80 | 20 | - |
Abbreviations: DMEM, Dulbecco’s modified eagle medium; FBS, fetal bovine serum.
3.3. Fast and slow Thawing Procedure
Fast thawing procedure: After one week, fast freezing procedure testes were removed from the nitrogen tank and in 30 seconds, above space them were filled with DMEM (Dulbecco’s modified eagle medium), under the hood, and rapidly was transferred into the water bath 37°C until defreeze. Then samples were transferred to its proprietary solution 1, 2 and 3, respectively (any solution for 5 minutes). Then were transferred to its proprietary solution 4 and were maintained for 30 minutes in the incubator (37°C).
3.4. Slow Thawing Procedure
Slow freezing procedure testes were removed from the nitrogen tank and were maintained at -70°C for 24 hours, at -20°C for one hour and at 4°C for one hour, respectively. Then above space they were filled with DMEM, under the hood. Other stages were taken such as fast thawing procedure [15].
3.5. Light Microscopy
Samples were removed from the incubator and were fixed in Bouin’s solution, dehydrated in ethanol, cleared in xylene, embedded in paraffin. Five sections (4 µm) from each testis were cut at intervals of 20 µm and stained with hematoxylin and eosin. Histological examinations were performed using a conventional light microscope (Diaplan, Leica Microsystem). Serial digital images were recorded at magnification of 400x.
Histological characteristics of the frozen-thawed testes were compared with of control testes. Slides were coded for blinded analysis by one person and counts were performed by two other observers. The integrity and the structural changes of controls and frozen-thawed sections were evaluated semi-quantitatively.
Nuclei of intratubular cells (spermatogonia and Sertoli cells) were scored as follow: i, distinction between Sertoli cells and spermatogonia nuclei was scored as 0 if easy, 1 if difficult and 2 if impossible; ii, observation of nucleoli was scored as 0 if easy (visible in 40% of cells) and scored as 1 if indistinguishable (in the case of pyknotic nuclei present in a large number and very condensed); iii, nuclei condensation was scored as 0 if absent or present in only 1 nucleus, as 1 if < 40% of nuclei were condensed and as 2 if > 40% were pyknotic. Therefore, a total absence of nuclei alteration was scored as 0 and the worst score for nuclei morphology was 5. The epithelium (constituted by intratubular cells) was scored as follow: i, detachment of cells from the basement membrane was scored as 0 if absent, as 2 if partial and as 3 if total or observed on > 75% of the circumference; ii, gap formation and shrinkage were scored as 0 if absent, as 1 if slight and as 2 if more obvious. Therefore, as for nuclei, epithelium morphology was scored from 0 to 5. The global score for each seminiferous cord section was the sum of nuclei and epithelium morphology and consequently was between 0 and 10. For each testis, the global score was the mean of scores for 20 seminiferous cords sections [15].
3.6. Statistical Analysis
Results (seminiferous tubuls injury) of treated groups with vitamins and control group were compared with Mean-Whitney U test, Kruskal-Wallis test and SPSS-22 software. Results are presented as Mean ± SD and statistical analysis were considered significant at P = 0.05
4. Results
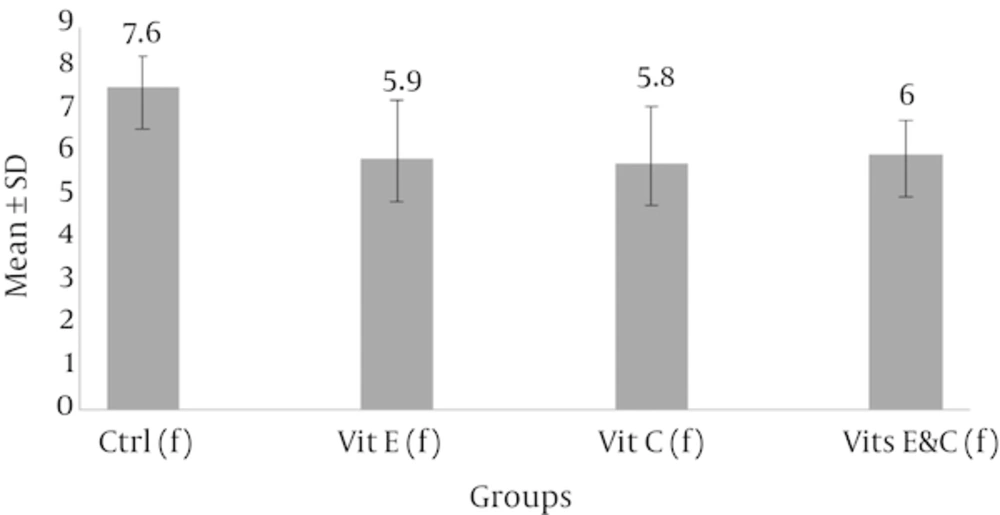
Fast freezing-thawing procedure: The results of Mann Whitney U test showed that injuries of seminiferous tubules had a significant decrease in each of the three experimental groups compared with of control group (P < 0.05). Despite the differences between the mean scores in the three experimental groups, Kruskal-Wallis test showed no significant difference between the three groups.
Abbreviations: Ctrl, control; Vit C, vitamin C; Vit E, vitamin E.
aSignificant differences compared with of control group.
b(f): Fast freezing-thawing procedure.
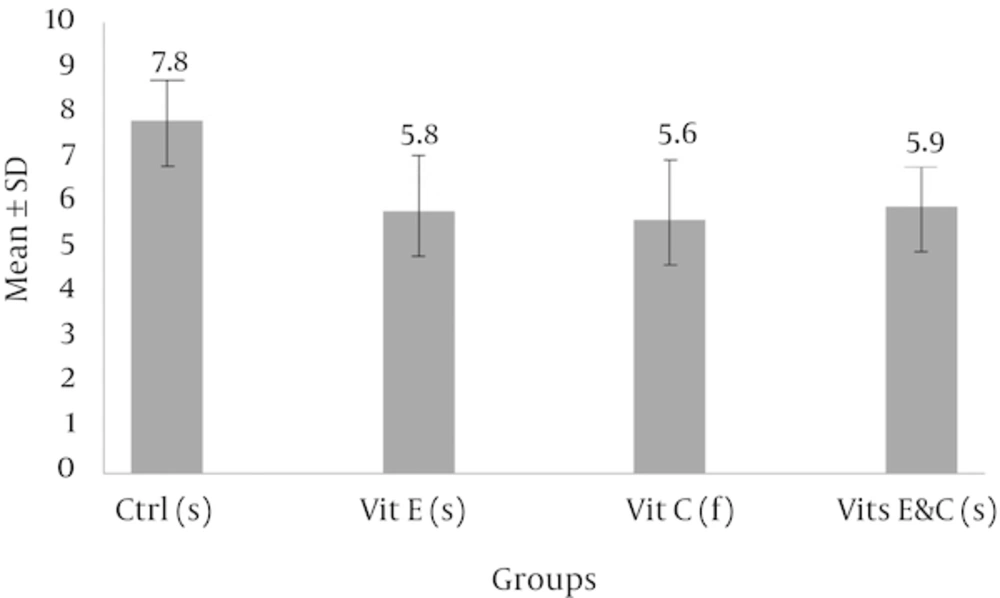
Slow freezing-thawing procedure: The results of Mann Whitney U-test showed that injuries of seminiferous tubules had a significant decrease in each of the three experimental groups compared with of control group. Despite the differences between the mean scores in the three experimental groups, Kruskal-Wallis test showed no significant difference between the three groups.
Abbreviations: Ctrl, control; Vit C, vitamin C; Vit E, vitamin E.
aSignificant differences compared with of control group.
b(f): Slow freezing-thawing procedure.
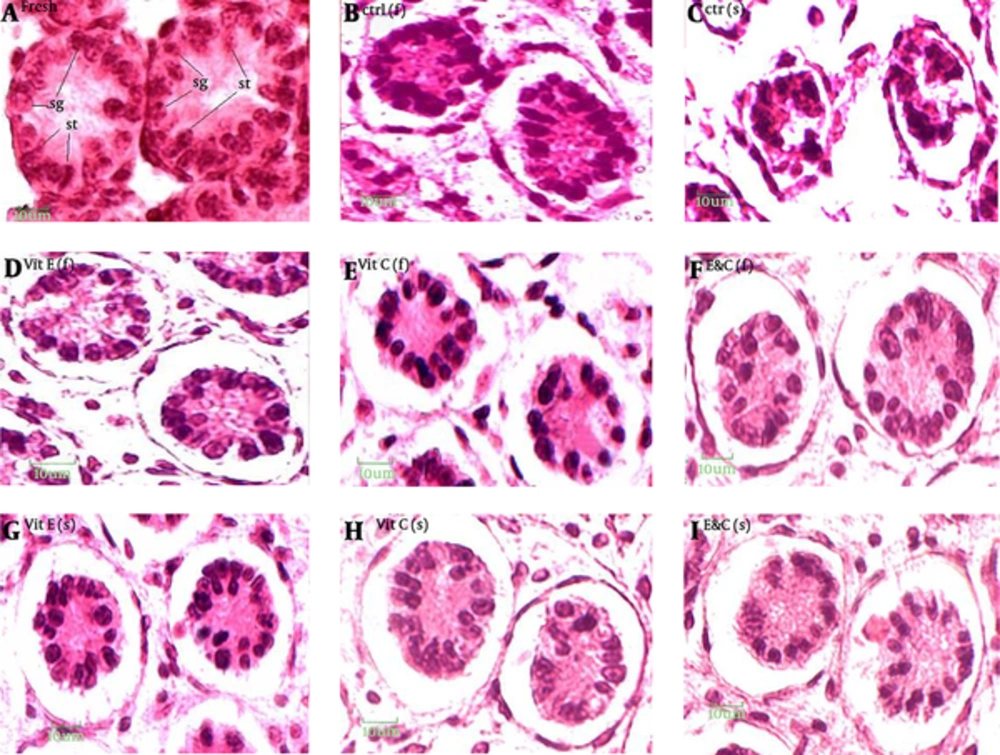
Scale bar: 10 µM. Fresh (A group of testes that in order to taking pictures, were fixed immediately after remove from mice): distinction between Sertoli cells and spermatogonia and observation of nucleoli is easy, nuclei condensation, detachment of cells from the basement membrane and gap formation and shrinkage is not observed. ctr (f), ctrl(s): distinction between Sertoli cells and spermatogonia and observation of nucleoli is impossible, nuclei condensation, detachment of cells from the basement membrane and gap formation and shrinkage is observed. Experimental groups: distinction between Sertoli cells and spermatogonia is difficult, observation of nucleoli is impossible, nuclei condensation is observed in some of nucleus but not all, detachment of cells from the basement membrane is observed, gap formation and shrinkage is not observed.
5. Discussion
Undoubtedly, encountering the cells and tissues with high concentrations of cryoprotectants and damages during freezing-thawing process, can cause to a lot of damages. During the freezing-thawing process the part of the enzyme systems that are involved in making normal free radicals are destroyed [16], ice crystals formation, osmotic shock, heat shock and oxidative stress are some of these damages. For example, there are still no good results in protection of stem cells after cryopreservation of testis due to the presence of free radicals [17], or ROS can damage membrane or membrane processes, lysis of cell, organelles dysfunction and abnormalities in the amount of calcium ions [18]. In mild or moderate oxidative stress occurrence, tissues often counteract its effect, but in the case of severe oxidative stress, the cells injury and may lead to cell death. In necrosis and apoptosis, free radicals are the major cause of cell death and in fact anti-apoptotic genes code a number of free radical neutralizers [19]. So there is essential need for strategies to reduce damages and substance cytotoxicity in solutions, for example many researchers believe that to combat the harmful effects of cryopectans and free radicals and oxidative stress, freezing-thawing, environments must be optimized [17] and they believe that cell type and the kinds of solutions effectively affect on the quality of freezed-thawed cell [20]. The results of this study showed that adding vitamins E and C to the freezing-thawing environment, separately and combined, in both fast freezing-thawing and slow freezing-thawing methods causes to reduce seminiferous tubes injury (layer of epithelial cells and the basement membrane) than what is observed in control group. These results are consistent with Yousef findings regarding the positive effects of vitamin E on the quality of frozen sperm of bull [21]. Other researchers have shown that vitamin E can prevent oxidative reactions by freezing protection of sperm [22].
In the present study, the effects of vitamin C are consistent with the results of a research by Asadpour et al. regarding the positive effects of adding vitamin C to freezing-thawing environment of sperm of a bull [16] and with the results of Yousef et al. that were announced the increase of motility, maturation, and survival of the sperm of a rabbit after adding ascorbic acid [21]. Although it does not conform the results of the research of Nazme-Bojnordi et al. that suggested adding vitamin C to sperm parameters is not meaningful [23]. The necessity for supplemental antioxidants to combat oxidative stress during freezing-thawing processes in addition to the conclusions of recent study, can also be inferred from the findings of Nair et al. evaluated and compared the percentage of the membrane oxidation and the activity of antioxidant enzymes in sperm of a bull and a buffalo during the freezing process. The activity of malondialdehyde enzyme that is the standard for oxidation of the membrane of the sperm, had increased, but the activity of antioxidant enzymes decreased in the sperm due to the high intensity of oxidative processes [22].
Generally, in the present study, the maximum effect of antioxidants was on preventing of gap formation and shrinkage and their effect on three indexes distinction between Sertoli cells and spermatogonia nuclei, observation of nucleoli and nuclei condensation was positive in some samples and was not found in other samples. In this study, formation of vacuole and shrinkage of the layer of epithelial cells is clearly seen in the control group, but the experimental groups did not show this damage at all or rarely show. This vacuole can result in a loss of cellular connections or reduction of adhesive molecules such as cadherin or loss of generative cells [24]. Peroxidation of poly unsaturated fatty acids of the membrane disturbs the function and permeability of the membrane, resulting in cell destruction and death. Since the main activity of vitamin E is to protect the polyunsaturated fatty acids chain in the cell membrane [25], can explain the positive effect of it in fighting against vacuole formation in this study. On the other hand, this vitamin might prevent the damage growth in cell DNA and expressing the responsible genes for its death during its frozen storage by reducing the production of free radicals [26]. Vitamin C might also be able to manage to protect lipoproteins against oxidative damage by various mechanisms such as preventing superoxide anion and oxygen species and entering other antioxidants into the cycle [14, 27], or maybe the low toxicity of vitamin C and its solubility in water has caused it to have protective effects as an additive antioxidant when it is add to the Cultivation environment [14].
The positive effects of vitamins on the nuclei condensation, distinction between Sertoli cells and spermatogonia nuclei, and observation of nucleoli indicators, probably derives from the fact that free radicals can cause a lot of damage to vital cell organelles and macromolecules of fauna such as proteins, lipids and carbohydrates during freezing-thawing process and affect on the synthesis of DNA and RNA and it is likely that oxidative stress acts in the same way in the case of generative sexual cells and disrupt their division and differentiation [28, 29]. But antioxidants have been able to combat against oxidative stresses imposed on nuclei and nucleoli with mechanisms such as oxygen removal or decreasing the topical concentration of oxygen, removal of active species such as superoxide and hydrogen peroxide and cutting the chain reactions. The minimum effect of antioxidants was on preventing of detachment of cells from the basement membrane. Detachment of cells from the basement membrane has completely occurred in experimental groups as in control group. There can be many reasons and possibilities in connection with this lake of effect. This lack of effect of antioxidants on recent index is maybe due to their inappropriate dose because the optimum function of antioxidants is achieved in certain concentrations [30, 31]. Perhaps the effect of antioxidants on mentioned index could be observed by selecting more appropriate doses or perhaps the lack of effectiveness of the latter case is due to the fact that freezing-thawing process in addition to oxidative stresses, other stronger pressures and factors are involved in detachment of cells from the basement membrane and thus antioxidants have not been able to fight it alone. There are also several possibilities about the amount of relatively similar effect of vitamin C or E and C and E; for example, the selected dose in combined mode was not suitable and therefore could not have been more effective than separate mode because the optimum function of antioxidants is achieved in certain concentrations [31], or maybe oxidative damages of this study and in these conditions have been in certain extent that each of these vitamins has had the potential to combat it and has dealt with it. Therefore, these two vitamins together have not had any synergistic effect.
Optimization of freezing-thawing environment by vitamins E, C and E and C, will decrease the harmful effects of fast and slow freezing-thawing process and will significantly protect the seminiferous tubes of immature testis from oxidation damages of this process. Comparison of the effects of vitamin E, C and E and C do not show any significant differences.