1. Introduction
WHO (world health organization) has reported that the malaria incidence rate and malaria death rate was 13 (per 1000) and 3 (per 100,000) in western Asia respectively. It was also anticipated that malaria will have caused 214 million cases (uncertainty range: 149 - 303 million) and 438,000 malaria deaths (range: 236,000 - 635,000) in 2015 [1]. The malaria mortality rates into growing ages have decreased by 60% [2]. P. vivax malaria occurred in the WHO eastern mediterranean region (11%). Globally, in 2015 the overall of malaria deaths due to P. vivax was estimated between 1400 and 14900. Iran has reduced malaria incidence rates by > 75% and it classify in the elimination phase since 2010 [3]. People suffer from Malaria as a major problem and economic issue in the south and southeast of Iran. These areas include the provinces of Sistan and Baluchistan, Hormozgan and Kerman [4, 5]. The rest of south of Iran are involved in leishmaniasis approximately [6]. Among 247 child cases from Sistan and Baluchistan province that registered as malaria patients, P. vivax was 83.8% of all species of the plasmodium (2013 - 2016) [7]. All malaria-positive cases were 287 as well as annual parasitic index (API) reported 0.18 (per 1000) in this province (Zahedan, Konarak, Saravan, Chabahar, Mirjaveh, Khash and Mehrestan) in 2015 [8]. Malaria control activities have been integrated in primary health centers of Iran since 1988 [9]. Resistance of Anopheles stephensi to DDT was first recognized in 1958 [10] and subsequently to dieldrin in 1960 [11] and Malathion in 1976 [12, 13]. Many adulticides e.g. propoxur from 1978 to 1993 for 13 successive years have been used for malaria control in the south of Iran [14]. Currently, the two most common vector control interventions are long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) [15]. Pyrethroids are particularly suitable for veterinary and public health purposes because of their quick knockdown effects, high insecticidal potency and relatively low mammalian hazard at operational doses [16, 17]. In recent years, pyrethroids have been used for residual spraying in malaria control programme. Lambdacyhalothrin was introduced in anti-malaria approach from 1992 [18]. Some investigations showed An.Stephensi was fully susceptible to malathion and pyrethroid insecticides but resistant to DDT and tolerant to dieldrin in south of Iran [19] also it was shown first report of pyrethroid resistance in An.stephensi in southern Iran [20]. The appearance of insecticide resistance is serious challenge. Scrutinize national anti-malaria strategy can help us to ensure that adoption of tools are promoted in implementation of priority methods. Periodic assessment of vector behavior and insecticide susceptibility status should be given in combating malaria [21]. One hundred and sixteen million people were protected by IRS in 2014 of the 43 countries had used pyrethraids for spraying in many parts of world [22]. The current research was conducted to evaluate the residual effects of two pyrethroid insecticides (lambdacyhalothrin, and deltamethrin) against adult female mosquitoes of Anopheles stephensi Liston (Diptera: Culicidae) on two surfaces (plaster and cement) in Saravan county as malariaous spot with annual parasitic index (API) of 0.75 and 0.43 (per 1000) within 2014 and 2015 respectively. As regards malaria due to P. vivax has accounted for around 89.1% of all cases in 2015 [8]. Accordingly, the result of this survey can be used in control management of malaria with focus on the role of IRS in Iran.
2. Methods
2.1. Study Site
The cross-sectional study was carried out from May until August 2015 in Saravan county that is located in Sistan and Baluchistan province, southeast Iran, comprising of 175727 populations living in an area of 16096 km2. The geographical coordinates are 22o27′ N; 62020′ E at an altitude of 1165 m above sea level. It has 384 km common borders with Pakistan. The mean annual rainfall is 107 mm and average annual temperature is 22.9 °C. Relative humidity in this district is 29%. The climate is hot with arid seasons. Its winter is moderate and the most agricultural product is date in this region.
2.2. Conical Test
Bioassay tests carried out according to WHO method so called conical test [23]. lambdacyhalothrin 10% (0.03 mg a.i./m2) and deltamethrin 5% (0.05 mg a.i./m2) within wettable powder (WP) and a standard X-Pert Hudson® pump with 10 liter capacity and nozzle 8002 were used for spraying. Pressure of solution in pump was 25 - 55 pon/inch2 and outcome rate was 757 c.c/min. Two insecticides noted above were applied on different surfaces including plaster, and cement. All the prepared surfaces were not stained and were free from any unpleasant odors during trial. IRS performed against 3960 blood- fed adult females of An.stephensi (mysoriensis strain) (4 - 5 days old) that were reared in our insectarium of study area. The evaluation started from May 2015 and continued every 14 days until 4 months later that in this time mortality rate was decreased lower than 80%. We released 10 adult females with aspirators into any cone at 10 replicates (upper, middle and lower parts of walls) with 30 minutes exposure on treated surfaces as well as on the fresh surfaces as control. After that, mosquitoes were transferred into clean cup and maintained in a climatic chamber for 24 hours at 27°C ± 2°C and 80% ± 10% RH. The percentage mortality recorded after 24 hours. If mortality rates of control mosquitoes to be 5% - 20%, it should be corrected by Abbott’s formula. Data analyzed by Mann-Whitney (nonparametric test) using SPSS v22 statistic software. Based on this survey, the mortality rates of An.stephensi Liston (Diptera: Culicidae) were interpreted between selected surfaces and using insecticides in several times. P value of ≤ 0.05 was employed index for significant difference.
3. Results
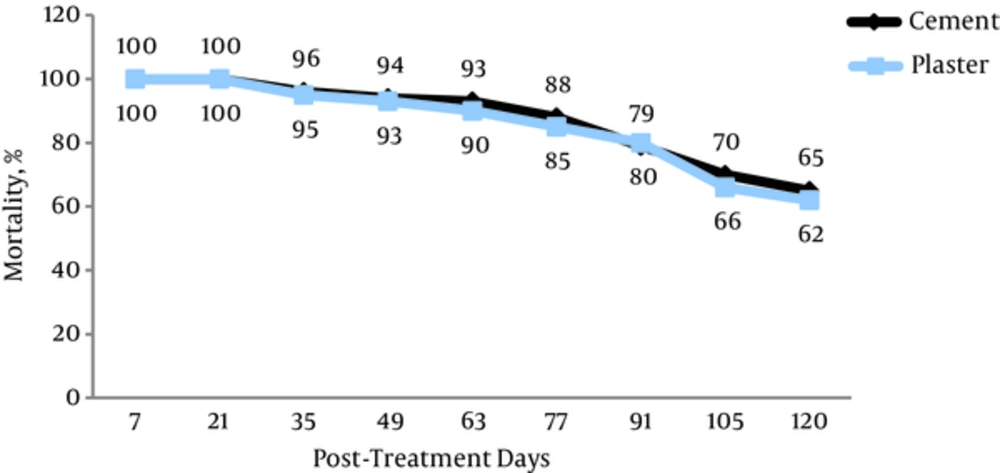
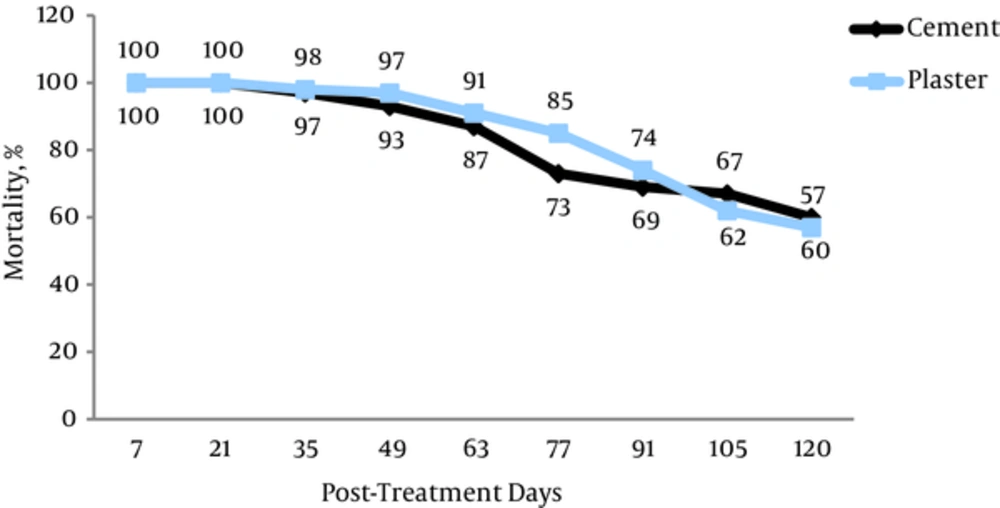
The results are shown in Figures 1 and 2. The mortality rate after 1 month for Lambdacyhalothrin was 96% ± 1.52 on cement surfaces and 95% ± 1.33 on plaster surfaces respectively. The mortality rate for deltamethrin was 97% ± 1.32 and 98% ± 1.62 on cement and plaster surfaces respectively. As the outcome is indicated that insecticides has 100% mortality rate within 2 posts-treatment weeks. 88% ± 1.00 and 85% ± 1.44 of mortality observed in the 77 days of spraying of lambdacyhalothrin on surfaces (cement and plaster). 85% ± 1.66 and 87% ± 1.32 reductions of Anopheles stephensi mosquitoes was shown in 77 and 63 days after deltamethrin spraying respectively. The full scale susceptibility was for first month posterior spraying in insecticides (lambdacyhalothrin and deltamethrin). We see 70% ± 1.52 and 66% ± 1.32 scale down in the abundance of Anopheles in 105 days posterior spraying on cement and plaster for lambdacyhalothrin while these values were 67% ± 1.00 and 62% ± 1.00 for deltamethrin respectively. P value related to lambdacyhalothrin between cement and plaster surfaces was 0.72 and for deltamethrin was 0.46. There was no significant difference between applied pesticides on cement surfaces (P = 0.12) within spraying times. Furthermore, on plaster wall there was no significant relation in lambdacyhalothrin compared to deltamethrin (P = 0.41). According to our finding the mortality rates of Anopheles mosquitoes have gradual decline from 100 to 65% ± 1.00 on cement wall after 4 months of field trial and it decrease towards 100 to 62% ± 1.33 on plaster wall at the same time for lambdacyhalothrin WP 10%. Meanwhile, the mortality rates of control groups in this paper were < 5%.
4. Discussion
Our knowledge has presented that the persistence of lambedacyhalothrin was up to 13 weeks on different surfaces whereas the residual efficacy of bendiocarb was recorded for 8 weeks against An.culicifacies in Indian trial [24]. To our investigation there was no significant relation on sprayed surfaces (cement and plaster) between lambdacyhalothrin and deltamethrin but lambdacyhalothrin had a slightly more durability than deltamethrin until causing 80% adult mortality (Figure 1). Similar results were documented in previous studies [25-28] thus we recommend lambdacyhalothrin to control malaria with two rounds of spray at an interval of 3 - 4 months in endemic area accordingly. Many researches were undertaken based on the susceptibility of vectors caused malaria disease to control and prevention of resistance in insecticide. Anopheleses were shown tolerances to some pesticide and resistance to certain compounds that were used throughout the world. Malaria was rampant due to resistance to deltamethrin in Anopheles funestus populations in south of Africa [29]. Afterwards, this species demonstrated resistance to permethrin, deltamethrin and D.D.T as has shown full susceptibility to bendiocarb and dieldrin in Uganda [30]. Also Anopheles epiroticus presented resistance to pyrethroids insecticides in some parts of Vitname, in Asia [31] as well as Anopheles maculates depicted tolerance to lambdacyhalothrin and permethrin [32]. Anopheles stephensi is the main vector of malaria in Iran [33]. Many different of insecticides has examined to determine resistance in Iran where malaria is still as a challenge. These studies led to using pyrethroids instead of others pesticides in health-care programme by ministry of health [34, 35]. In Iran, bioassay tests were conducted on these pesticides (lambdacyhaothrin, cyfluthrin and deltamethrin). More than 95% of Anopheles stephensi populations were displayed susceptibility. We applied 0.05 mg/m2 of lambdacyhalothrin but Ladoni et al. evaluated the residual effect of lambdacyhalothrin (ICON 10% WP) at varied dosages on treated surfaces in south of Iran. Icon at 50 mg/m2 had a residual activity for more than 4 months on cement and plaster [25]. Moreover, in a bioassay test was examined (2004 - 2005) the residual impact of deltamethrin 5% at 25 mg/m2 was 3 months on target surfaces (plaster, mud, wood) versus main vector in Sistan and Baluchistan province in the same local of our survey [36]. In comparison with the finding, the irritancy of pesticides on varied surfaces responses for this difference probably. The casual contact with a surface treated for pyrethroids can provide an irritant effect, causing the insect to escape. This impact has been known as excito-repellency [37]. A new formulation was used in other survey and the maximal residual time of deltamethrin WG 25% (25, 40 and 50 mg/m2) was obtained 2, 3 and 4 months. It seems that the formulation (WP) was applied in our study had higher residual time [38]. Climatic factor such as humidity and temperature can affect the activity of insecticide. Then the insecticide durability was evaluated on simulated surfaces in laboratory conditions by Vatandoost et al. Based on this research the persistence of deltamethrin was shown about four months on plaster (mortality = 77 ± 6.2%) and 4.5 months on cement (79 ± 3.2%) [39]. The outcome of a research in Hormozgan province that was subjected as a major foci of malaria infection showed that the residual effect was up to 4 months using cyfluthrin (Solfac WP 10%) at 50 mg/m2. This residual activity was in accordance with our assessment [40]. Pyrethroid insecticides have been tested on nets and revealed acceptable effect. For example, efficacy of Lambdacyhalothrin (SC 10%) -impregnated bednets was measured around 4 months to mortality rate was dropped lower than 90% on different anopheles species in Siaho (Bandar Abbas) [41]. It is noteworthy that the residual activity of pyrethroids was estimated about 2 - 4 months on type of surfaces in evaluated studies. Entirely, variation in species and behavioral traits, climatic factors, difference in concentrations of insecticides, formulation, sprayed surfaces, type of feeding (indoor or outdoor) and social acceptability can affect the durability of insecticides [39, 42]. Applying IRS in combination with ITNs (insecticide- treated nets) provided protective effect compared to using either interventions alone [43]. As the coverage percentage of ITNs rised, the incidence rate of malaria began to decline in Sarbaz county, southern Iran [44]. There are many reasons for this application. IRS + ITN reduced the risk of malaria disease and put off the emergence of insecticide resistance because of using different classes of insecticide for IRS and ITN in areas where the infection is endemic [45]. Besides indoor residual spraying likely has restrictive effect in some parts where P. vivax is rampant, vector feds and transfers parasite early in the evening outdoors [46]. Therefore we should consider implementing IRS with ITN in the seasonal peaks and outbreak times to elimination of malaria more rapidly in southeastern Iran in spite of resistance.