1. Background
Multipotent differentiation, anti-inflammatory, and immunoregulatory potentials are the properties that make mesenchymal stem cells (MSCs) an excellent candidate for cell therapy (1, 2). However, although the results of many in vitro studies are favorable, outcomes of animal models or clinical trials may not be that much satisfactory (2). Bone marrow MSCs have a good proliferative potential as well as the advantage of being autologous sources, but there is some hindrance to its wide range of therapeutic applications (3-5). First, the absolute number of MSCs significantly decreases after repeated passages, which is a limiting factor hampering its clinical application. Secondly, advanced age of MSCs due to successive passages or aged donors reduces proliferative potential (6). Studies prove that consecutive in vitro proliferation of MSCs leads to the telomere length reduction and reduced telomerase activity (7). In addition, changes in gene expression of aged MSCs lead to impaired cell activation, migration, and production of anti-inflammatory molecules (8).
Estrogen affects many physiological processes in mammals such as cellular proliferation, apoptosis, differentiation, and metabolism to name, but a handful (9, 10). It is demonstrated that 17 β-estradiol (E2) can effectively advance bone marrow stromal cell proliferation and differentiation in rodents and human (8). On the other hand, estrogen reduces the apoptotic rate in MSCs, actually, E2 upregulates the expression of Bcl-x(L) and Bcl-2, which play critical anti-apoptotic roles (11). Also, studies suggest that estrogen adequate dosage can slow telomere shortening rate in treated cells (12). Altogether, it seems that E2 has all the desirable properties of a potent supplement to improve MSCs expansion and function and makes MSCs application in animal models more efficiently.
Ulcerative colitis (UC) is a different form of inflammatory bowel disease (IBD). This disease is characterized by diffuse inflammation in the colonic mucosa (13). The advent of stem cell research unveiled the therapeutic potential of these cells to treat several inflammatory diseases including UC to name, but a few. According to the available literature, MSC is a potent candidate to harness IBD and restricts its clinical signs (14-16).
2. Objectives
By the utilization of a UC model in healthy male rats, the current study herein examined how estrogen could affect MSCs function in colon tissues with inflammation.
3. Methods
3.1. Reagents
The ELISA (enzyme-linked immunosorbent assay) kits were purchased from Peprotech (UK). Total protein assay kit was obtained from Zist Chemi Co. (Tehran, Iran). Other reagents were purchased from Sigma-Aldrich Company (St. Louis, MO).
3.2. Animals
The male Wister rats were purchased from Experimental Animal Care Center of Urmia University. The animals were kept under controlled environmental conditions (12:12 hours light/dark cycle at 25°C). Also, animals received food and water ad libitum. All the experimental procedures were conducted in accordance with the regulations of the Ministry of Health and Medical Education of Iran, approved by the Ethics Committee of Urmia University.
3.3. Induction of Colitis
Colitis was induced in rats as described earlier (17). At first, the animals were fasted for 36 hours. Then, the animals underwent a light ether anesthesia and a pediatric catheter was placed into the colon so that the tip of the catheter was 8 cm proximal to the anus. A 2-mL solution of 4% acetic acid or saline alone (in normal rats) was introduced to the colon. Rats were held in head down vertical position for one minute to impede acetic acid leakage.
3.4. Experimental Groups
One hour after induction of colitis, rats were randomly distributed into the four groups of 10 as the control colitis, MSCs-treated, E2-primed MSCs-treated (E2-MSCs), and normal. The average weight of rats in all groups at the beginning of the study was 284.1 ± 0.94 g. Normal rats underwent the same procedure of colitis induction, but received an equal volume of normal saline instead of acetic acid and underwent no treatment. Ratsin the control colitis group received only the vehicle (400 μL of phosphate-buffered saline (PBS) intraperitoneally). The third and fourth group of animals were respectively received MSCs or E2-MSCs (2 × 106 cells for each rat). Therapies were started on the day of acetic acid instillation until the day 10, when rats were sacrificed.
3.5. Isolation and Expansion of MSCs
Bone marrow-derived MSCs were isolated and expanded as a routine according to the previously described method (18). Briefly, flushed out marrow was washed and centrifuged in PBS and cells were plated in 75-cm2 tissue-culture flasks at the concentration of 0.4 × 106 cell/cm2 in DEMEM (Dulbecco’s modified eagle medium). Four days after primary culture, culture medium was exchanged and adherent cells were fed every other day. Upon 70% confluence, cells were trypsinized, counted, and passed at 1:3 ratios. The cell suspension of the third generational passage was collected and incubated with concentration of 0 or 100 µM E2 for 24 hours before harvesting.
3.6. Charecterization of MSCs
The isolated cells in subculture 3 were used for characterization in accordance with a previous work (19). The fluorescent-conjugated monoclonal antibodies; i.e., PE-labeled anti-CD29, FITC- labeled anti-CD45, and PCY5-labeled anti-CD90 were exploited for MSC markers staining.
Regression of the polyclonal T-cell proliferation is one of the important hallmarks of MSCs (20). Splenocytes were aseptically separated and stimulated with phytohemagglutinin or medium alone and incubated for five days. The MTT assay was performed as a routine according to the previously described method (20).
3.7. Evaluation of Animal’s Well-Being
Disease activity index (DAI) was estimated as the sum of scores of stool consistency, blood feces, and weight loss, and according to the parameters outlined in Table 1.
| Score | Weight Loss, % | Blood Feces | Stool Consistency |
|---|---|---|---|
| 0 | Negative | Negative | Normal |
| 1 | 1 - 9 | Red | Soft |
| 2 | 10 - 19 | Dark red | Very soft |
| 3 | < 20% | Black | Diarrhea |
aThe disease activity index was calculated as the sum of scores of all criteria.
3.8. Colonic Homogenate
Ten centimeters of distal colon was removed, freed from surrounding tissue, opened longitudinally, and rinsed with PBS. Tissue was homogenized in 10 volumes of ice-cold saline buffer. The homogenized specimen was centrifuged at 10000 g for 10 minutes at 4°C (21).
3.9. Assessment of Biochemical Changes in the Colonic Homogenate
Myeloperoxidase (MPO) activity in the colonic homogenate was determined by a similar procedure described earlier (22). MPO activity was calculated as the difference of the absorbance relating to the horseradish peroxidase standard curve. To check nitric oxide concentration in colon tissue, the Griess method was applied (23). Lipid peroxidation was determined by measuring malondialdehyde similar to the procedure described earlier by Al-Rejaie et al. (24). To assess total protein concentration in the colonic tissue, the pyrogallol red-molybdate method was used (25).
The levels of IL-6, IL-1β, and TNF-α in the colon homogenates were estimated by a commercially available ELISA kit, according to manufacturer’s instructions.
3.10. Statistical Analysis
Continuous values were subjected to a one-way analysis of variance (ANOVA) plus the Dunnett post hoc test. For the discontinuous ranks related to the severity of the disease, the Kruskal-Wallis test was used. Obtained data were expressed as mean ± standard error of the mean (SEM). The P values of less than 0.05 were remarked statistically significant.
4. Results
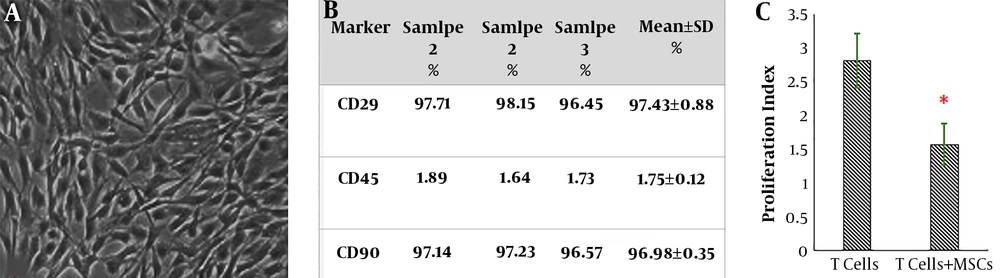
Bone marrow-derived adherent cells gradually formed small colonies; therefore, a homogeneous fibroblast-like, spindle-shaped morphology was appeared in the subculture 3 (Figure 1A). Flow cytometric analysis showed that CD 45 (the marker for the hematopoietic cell) was not expressed in the sub-culture 3 of bone marrow-derived adherent cells, whereas both CD 29 and CD 90 (consensus markers for MSCs of rat) were clearly observed (Figure 1B). Data also indicated that isolated MSCs could suppress the proliferation of polyclonally stimulated T-lymphocytes (Figure 1C).
Characterization of mesenchymal stem cells. A, subculture 3 of cells depicted large, flattened or fibroblast-like morphology; the typical shape of MSCs (X40); B, the immunophenotypic characterization of MSCs by flow cytometry; C, the results of co-culture of MSCs T-lymphocytes. *P < 0.01 vs. T-lymphocytes without MSCs.
As displayed in Table 2, acetic acid instillation into the colon of rats caused a high DAI score in the rats. Also, the mean weight of the rats with UC significantly reduced compared to the normal animals. The weight loss severity and DAI index were noticeably restricted in all of the treated animals. Additionally, gaining weight and reduction of the DAI were more profound in the E2-MSCs group than the untreated MSCs-treated rats (Table 2).
aThe values are expressed as mean ± SD.
bP < 0.001 vs. normal rats.
cP < 0.01 vs. the colitis group.
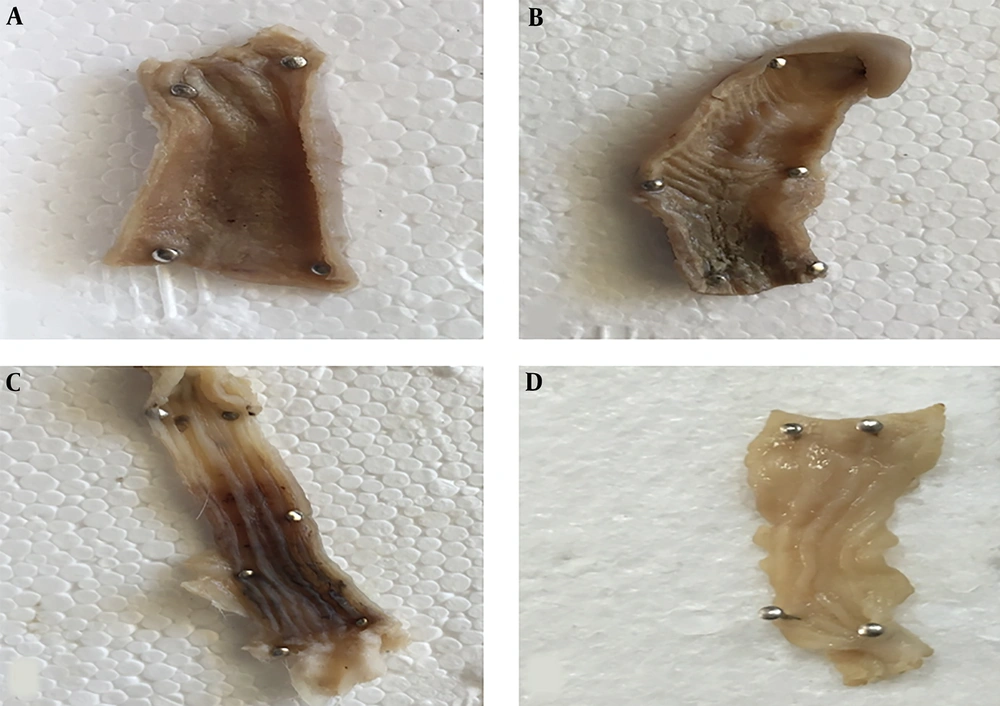
The macroscopic evaluation displayed widespread lesions along the distal part of the colon in rats in the control colitis group. Ulceration, edema, hyperemia, and hemorrhagic mucosa were obvious to the naked eye (Figure 2). The results showed a significant amelioration in both treatment groups compared to the rats in the control colitis group (Figure 2).
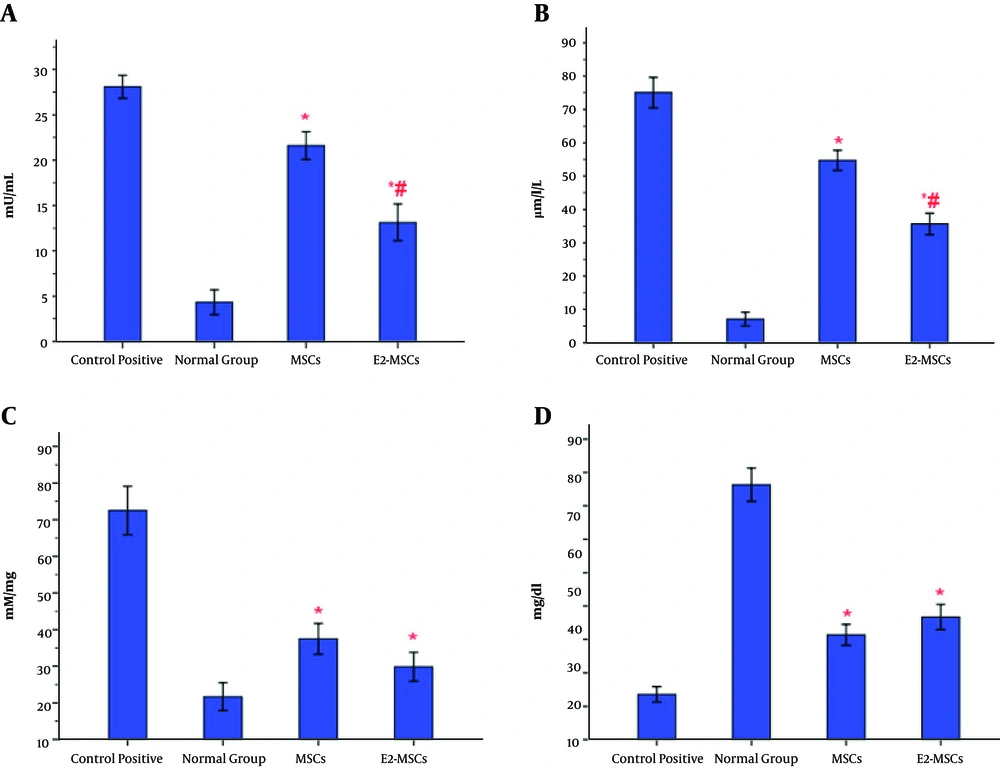
Cell therapies caused a remarkable regression in MPO activity, nitric oxide production, and tissue concentration of MDA in the colonic specimens compared to the colitis control group, which increased prominently (Figure 3A-C). Also, the level of total protein was enhanced in both treatment groups compared to control positive rats, which decreased significantly (Figure 3D). Albite, the result showed that MPO activity and nitric oxide levels reduced more significantly in E2-MSCs-treated rats than the ones that just received MSCs (Figure 3A and 3B). Additionally, there were no significant differences in MDA activity and total protein content of colonic homogenates between the E2-MSCs group and healthy rats (Figure 3C).
Evaluation of biochemical changes in the colonic tissue. The levels of myeloperoxidase activity (A) and nitric oxide (B) were downregulated in the gut of E2-MSCs-treated rats more than the untreated-MSCs group. Moreover, treatment with E2-MSCs and MSCs could decrease the level of MDA in acetic acid-induced colitis. Nevertheless, there were no significant differences in MDA activity between E2-MSCs groupand healthy rats (C). Also, both therapy could equally enhance the level of total protein in the colonic homogenate compared to control positive rats (D). *P < 0.001 vs. control positive rats; #P < 0.01 vs. MSCs-treated group.
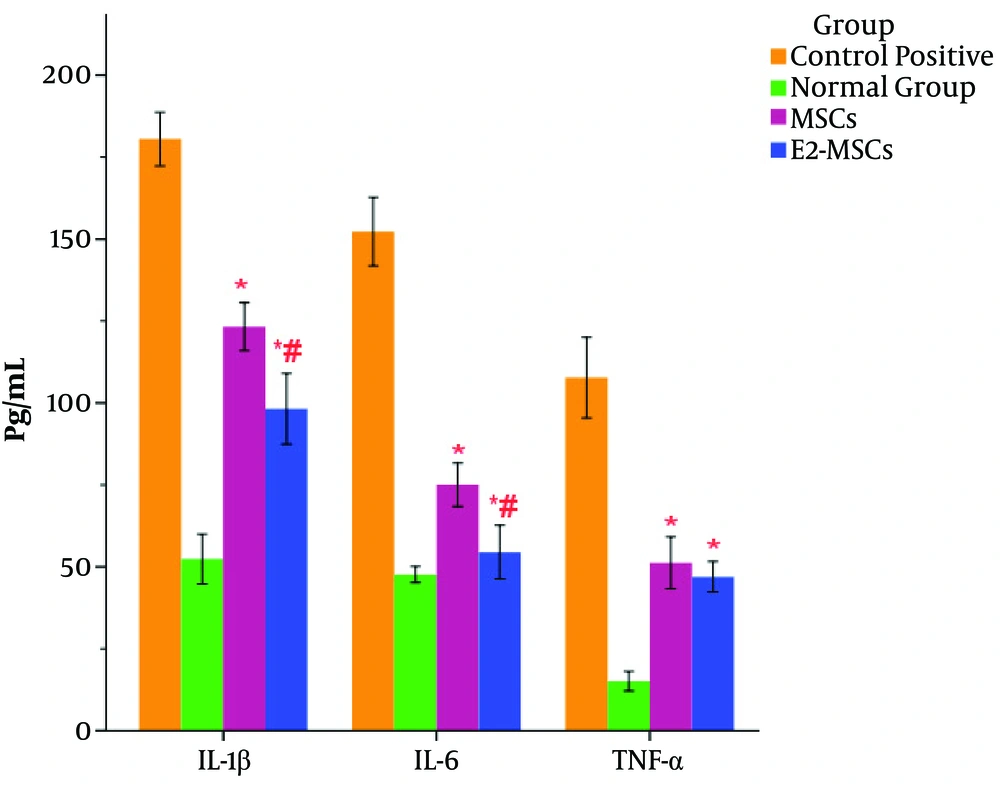
The production of the IL-6, TNF-α, and IL-1β significantly increased in rats with UC compared to normal ones (Figure 4). Both cell therapies caused a significant decrease in the production of the proinflammatory IL-6, TNF-α, and IL-1β cytokines in the colonic homogenate of animals with colitis compared to the vehicle-treated rats with UC (Figure 4). Albeit, cell therapy with E2-primed MSCs caused a significant reduction in IL-6 and IL-1β production compared to the rats with UC receiving untreated MSCs (Figure 4). Moreover, there were no significant differences in IL-6 levels between E2-MSCs-treated rats and healthy ones (Figure 4).
Assessment of cytokines profile in the colonic tissue. The levels of TNF-α, IL-1β, and IL-6 were reduced in both treatment groups in a significant manner compared to the control rats with colitis. Cell therapy with E2-primed MSCs caused a significant reduction in IL-6 and IL-1β production compared to the MSCs group. In addition, there were no significant differences in IL-6 levels between the E2-MSCs groups and healthy rats. **P < 0.001 and *P < 0.01 vs. the control positive rats; #P < 0.01 vs. the MSCs-treated group.
5. Discussion
The main obstacle against a wide clinical application of MSCs is the limited cell number and reduced differentiation potency. However, these limiting factors are unenviable, but MSCs potentials and characteristics outweigh them. Hereupon, many studies are conducted to find a strong stimulus that can improve MSCs proliferation and functions before administration (26-28). It is documented that when rats are maintained in a hypoxic environment for three weeks, a significant increase in the circulating MSCs (by almost 15-fold) could be observed (29). It is also clear that both hypoxia and E2 can induce hypoxia-inducible factor 1a (HIF-1a) in the target cells (30). A very interesting survey reported that E2 treatment of MSCs via induction of HIF-1a could facilitate migration of transplanted MSCs to the injured pancreas (31). Age and senescence are among the criteria modifying the potential of cell therapy and negatively influence MSCs’ differentiation and expansion (32). Therefore, it is necessary to maintain MSCs functions. Previously, Zhang et al. demonstrated that estrogen treatment possesses a very valuable effect on the improvement of MSCs differentiation and expansion in vitro (33). In addition, Zamani Mazdeh et al. reported that E2 augments the differentiation potential of MSCs and bone regeneration in a rabbit model of radial non-union segmental defect (34). Here, the current study results indicated that the E2-primed MSCs in the rats with UC promoted a more favorable sequel, creating the reversion of the clinical score and biochemical changes, which are more favorable than the therapy with untreated MSCs.
The influx of neutrophils into the colon possesses a critical role in the progression of tissue necrosis and inflammation (35). Neutrophils are the most prominent cells that infiltrate into the colonic tissue following acetic acid insulation. Aggregation of neutrophil and other emigrant cells cause potentiation and prolongation of inflammatory injuries by uncontrolled production of oxygen and nitrogen species (36). The proinflammatory nature of these reactive substances causes a remarkable recruitment of neutrophils to the inflamed site; however, unsaturated bonds among phospholipids molecules of cell membrane target the free radical species too. According to the literature, tissue concentrations of MDA increase in the murine models of colitis due to lipid peroxidation (37). Myeloperoxidase is the most abundant molecule detected in neutrophil granulocytes that produces hypohalous acids during the respiratory burst reactions. It causes tissue destruction by nitrosating, oxidizing, and chlorinating proteins. In the present study, both MDA and MPO concentrations were remarkably diminished in the E2-treated group compared to the untreated MSCs group. Additionally, decrease of MDA and MPO in colonic tissue was approved macroscopically by attenuation of damage scores. Reduction of MDA in E2-MSCs-treated specimens might be a result of decreased oxygen and nitrogen species level in colonic tissue. Interestingly, MPO usually observed in the azurophilic granules of neutrophils can be applied in an indirect way to estimate the neutrophil content of a tissue sample similar to colonic specimens in UC condition (38). Similarly, a significant reduction in nitric oxide concentration in the E2-treated group approves the study hypothesis regarding the estrogen efficacy on MSCs proliferation in vivo.
The inflammatory cytokines such as IL-6, IL-1β, and TNF-α intensify proinflammatory reactions and injuries due to their proinflammatory role (39). Thus, reduction of the proinflammatory cytokines could be a beneficial approach to restrain clinical signs of UC as far as the anti-inflammatory potency of the IL-1 receptor antagonist (IL-1ra) was documented in the animal model of ulcerative colitis induced by acetic acid in rats (40). The attained data showed that the levels of these cytokines reduced in the colonic homogenate of MSCs-treated animals. Nevertheless, E2-primed MSCs decreased the levels of IL-6 and IL-1β more profoundly than MSCs alone.
5.1. Conclusions
The current study results illustrated that MSCs primed with estrogen significantly decrease colonic damage, which is probably associated with the prominent reduction of lipid peroxidation, neutrophil infiltration, and colonic levels of proinflammatory mediators.