1. Background
The complex mainstream of “wound healing” is a process leading to contraction and closure of the wound and causing the retrieve of skin’s functional barrier (1). Various events, including inflammation, proliferation, and migration of different cell types contribute to damaged tissues repair (2). Wound dressing is a therapy to repair the injured skin. Recently, electrospun nanofibrous membrane has revealed high potential in the wound dressing field. The membrane adheres uniformly at the moist wound surface without accumulation of fluids (3). Wound dressing with electrospun nanofibrous membrane would provide gas permeability and protect the wound against infection and dehydration. To reach the aim of dressing as high wound protection, materials must be selected precisely and the structure must be confirmed to be a good barrier against contamination and has oxygen permeability properties (4). Polyvinyl alcohol (PVA) is a bioadaptable, nonvenomous, water-soluble, and biodegradable synthetic polymer (5), which is usually used in biomedical fields (6).
Natural products have played a key role in wound healing research and many medicines are either natual products or derivatives thereof (7). It is known that the wound-healing process can be eased by antioxidants, since these properties co-exist in many plant species from a broad spectrum of families. Pomegranate (Punica granatum L.) has been used widely in folklore medicine for centuries to treat or prevent a wide range of disease. The potential therapeutic properties of pomegranate peel are extensive, including prevention or treatments of cancer (8), cardiovascular diseases (9), diabetes (10), dental conditions (11), erectile dysfunction, protection from ultraviolet (UV) radiation (12), and antimicrobial activities (13). Other potential applications include infant brain ischemia, Alzeimer’s (10, 14), arthritis (2), obesity (12), and dermal wounds (13). It has been characterized by having phenolic compounds, such as flavonoids, including cathechins, anthocyanins and other complex flavonoids; and hydrolizable tanins like punicalagin, ellagic acid, gallic acid, unicalin, and pedunculagin. These compounds are detected in pomegranate peel and juice, and are responsible for 92% of the antioxidant activity related to the fruit (15). The goal of many pomegranate researches has been to isolate and characterize the therapeutic constituents.
Hydrolizable tannins, such as gallotannins and ellagitannin, break down on hydrolysis to release ellagic acid or gallic acid and glucose, respectively (16). Punicalagin has shown considerable pharmacological activities, for instance anti-inflammatory (17), antioxidant (18), antigenotoxic (19), and hepatoprotective (16) activities. Frequent oral administration of high dose of punicalagin in rats for 37 days was found to be non-toxic (20).
2. Objectives
Many studies have shown that pomegranate peel has wound healing properties (21), yet its pure punicalagin component was not studied. Punicalagin, unique to pomegranate as a part of ellagitannins family with a molecular weight of greater than 1000, is accounted for more than 50% of the potent antioxidant activity of the pomegranate peel (22). Given that the use of electrospinning as a wound dressing is on the rise, this study aimed at evaluating the effect of punicalagin nanofibrous wound dressing on antioxidant capacity index through wound healing in rats.
3. Methods
3.1.Nanofibrous Bandage
Punicalagin (as standards) and PVA (MW = 195000, degree of polimerization = 4300, and degree of hydrolysis = 98.0 - 98.8 mol %) were bought from Sigma-Aldrich, Germany. All standards and solvents were HPLC-grade. The other chemicals related to this research were purchased from the Merck chemical company (Darmstadt, Germany). Purification of punicalagin from pomegranate peel followed a published method with minor modifications (23). Fresh pomegranate pericarp (50 g) was blended, mixed with 250 mL water, and the suspension was kept at 25°C for 12 hours. After filteration water extract was dried on an evaporator at 50°C and remnant solid was re-dissolved in 20 mL water, then loaded on a 30 × 400 mm column packed with Amberlite XAD-16N resin and column was eluted with 2 L of water. Total pomegranate tannin (TPT) was recovered from the column using 400 mL methanol. Methanol elute was evaporated at 50°C to yield TPT as dry powder. A Sephadex lipophilic LH-20 resin column was used to isolate the pure compound from TPT. One hundred miligrams of TPT powder was suspended in Sephadex lipophilic LH-20 resin that was pre-equilibrated with H2O and eluted with stepwise increasing concentrations (0, 20, 40, 60, 80, 100%) of methanol to give six fractions. Each fraction was analyzed by HPLC. Before injection, samples were centrifuged at 5000 rpm for four minutes and the supernant was filtered through a 0.4-µm filter. A Knauer reversed phase HPLC system (Germany) with a 4.6 × 250 mm C18 separation column at 20˚C was used. The HPLC method was performed as described by Hayouni et al., (13), with minor modifications, in which two solvents were used in the mobile phase, including solvent A (0.1% formic acid) in water and solvent B (100% methanol). The gradient was as follows: Initially 1% B in A for five minutes followed by 1 to 20% B in A for 15 minutes, 20 to 40 % B in A for one minute, 40% B in A for five minutes, 40 to 95% B for five minutes, and finally 15% B for the next 15 minutes. Absorption was detected at 254 nm (13). Ellagitannin (punicalagins and ellagic acid)- rich fraction was evaporated and re-chromatographed on Sephadex lipophilic LH-20 column and eluted, this time with stepwise increasing concentration (0, 20, 40, 60, 80, 100%) of ethanol (six fractions). The punicalagin-rich fraction was filtered through 0.45-µm filter and 100 µm and was injected on the HPLC column.
Ellagitannin (100 mg) was absorbed on a Sephadex-LH-20 column, which was pre-equilibrated with H2O and eluted with increasing amounts of ethanol to give six fractions. The sixth fraction contained pure punicalagin. To detect punicalagin, its standard sample was used as external standard and then percentage of area was calculated.
Electrospinning was performed as follows: PVA: punicalagin solutions were prepared by dissolving weighted amounts of PVA and punicalagin at ratio of 8:20 in distilled water to prepare a 6% wt solution. The mixture was shaken to obtain a clear and homogeneous solution. Electrospinnng was performed using high-voltage DC power supply (RP50-1.25R/230DDPM, Gamma high-voltage Research, USA) and a syring pump (KD-100, KD Scientific, Holliston, MA) connected to a 5-mL syringe with a 0.7 mm inner diameter stainless steel needle. In preliminary experiments, electrospinning was carried out at 20 KV voltages, and 1 mL/h flow rate (at room temperature). The distance between needle tip and the collector was 20 cm. By scanning electron microscopy (SEM) (XL30ESEM FEG) the morphology of the electrospun fibers was assessed (24).
3.2. Animals and Experimental Protocol
All animal procedures were approved by the Laboratory Animal Center of Shiraz University. Overall, 250 ± 30 g adult Wistar rats were sheltered under pathogen free conditions in stainless steel cages, with cycle of 12 hours light and dark. On the operation day, rats were anesthetized by 0.8 mL 5% ketamine and 0.2 mL xylazine (IM injection). A 900 mm2 (3 cm × 3 cm) full-thickness rectangular wound in dorsal inter-scapular skin was incised. The duration of anesthesia was about 15 minutes for each rat.
After recovery from anesthesia, animals were divided to two groups, consisting of ten animals each (each group was divided to two equal subgroups of five rats to be euthanized at 7 and 20 day) and were returned to separate disinfected cages, individually. The first group was treated with nanofibrous without punicalagin and the second group was treated with nanofibrous with punicalagin, as dressing. The rats were kept at room temperature of 25 ± 2°C. The nanofibers dressings were applied once a day for seven consecutive days. During this period, the animals were gently handled to minimize the stress and to get them acclimatized to the laboratory environment.
On day seven and 20 after the operation, the entire wound area on each animal was cut out as samples to be evaluatd and stored at -70°C until analyses. Animals were finally euthanized.
3.3. Total Antioxidant Capacity (TAC) Measurement
For measuring total antioxidant capacity (TAC), Zell Bio Company (Germany) commercial kit was used. The color product of chromogenic substrate (tetramethylbenzidine) revealed and at 450 nm, the color change was measured calorimetrically and expressed as micromoles per litre tissue extract (μmol/L).
3.4. Statistical Analysis
Statistical analysis was done and comparisons between variables were assessed with SPSS statistics software (version 19). Significances were calculated with t-test, Analysis of Variance (ANOVA) and complementary Duncan tests by comparing total antioxidant capacity in group treated with punicalagin dressing versus controls. P value of less than 0.05 were considered as significant.
4. Results
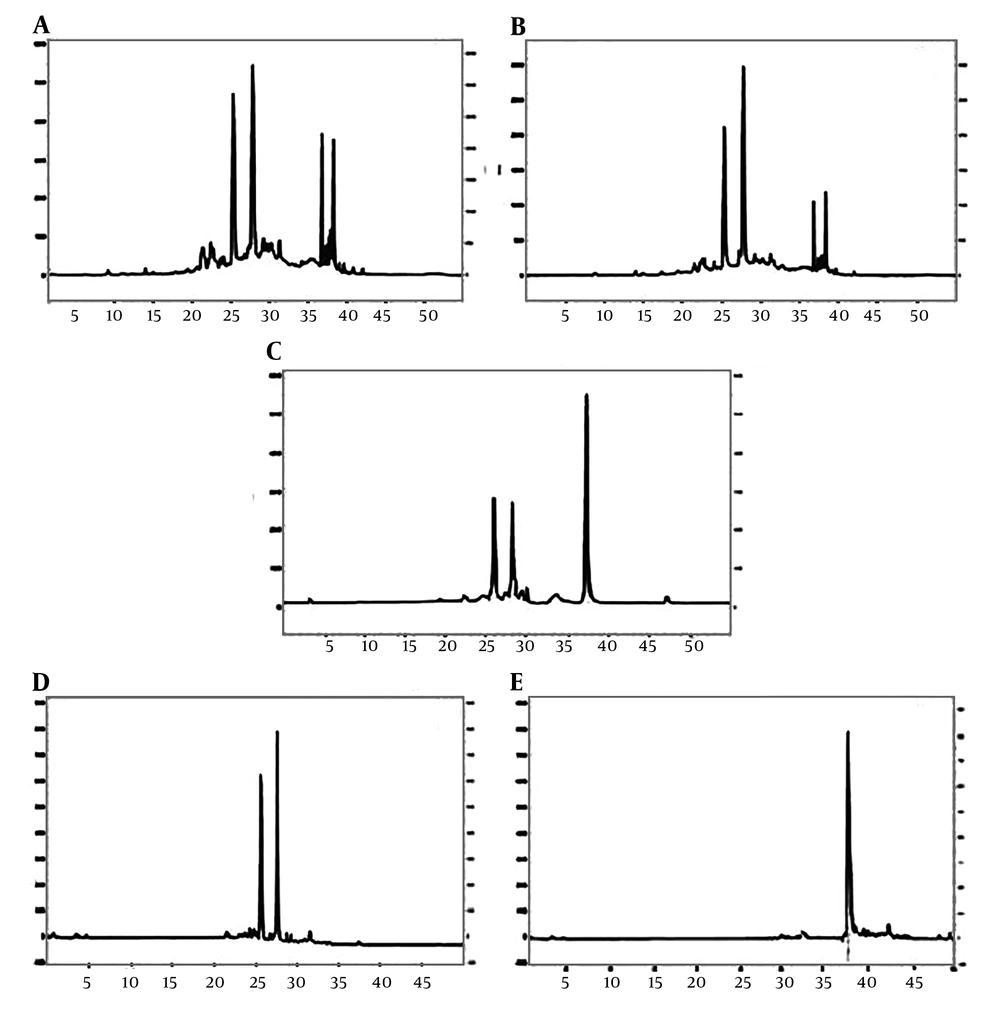
Figure 1A shows purification of punicalagin. Initially XAD16 column and 100% methanol were used for separating TPTs from pomegranate peel. The HPLC profile of TPTs showed four peaks corresponded to 4 ellagitanins (Figure 1B). Each peack was identified based on external standards. Peaks 1 and 2 were identified as alpha and bete punicalagin anomers and the 4th peack was ellagic acid. The TPTs contained 50% to 71% w/w of punicalagin anomers and 2% to 6% w/w ellagic acid. More purification was done by loading TPTs on LH-20 Sephadex column so that fraction ng at 60% to 68% methanol had the highest amounts of punicalagin and ellagic acid, (Figure 1C). Punicalagin and ellagic acid were separated from each other by reloading this fraction on the LH-20 column and increasing the gradient of ethanol, as described in materials and methods section, (Figures 1D and 1E).
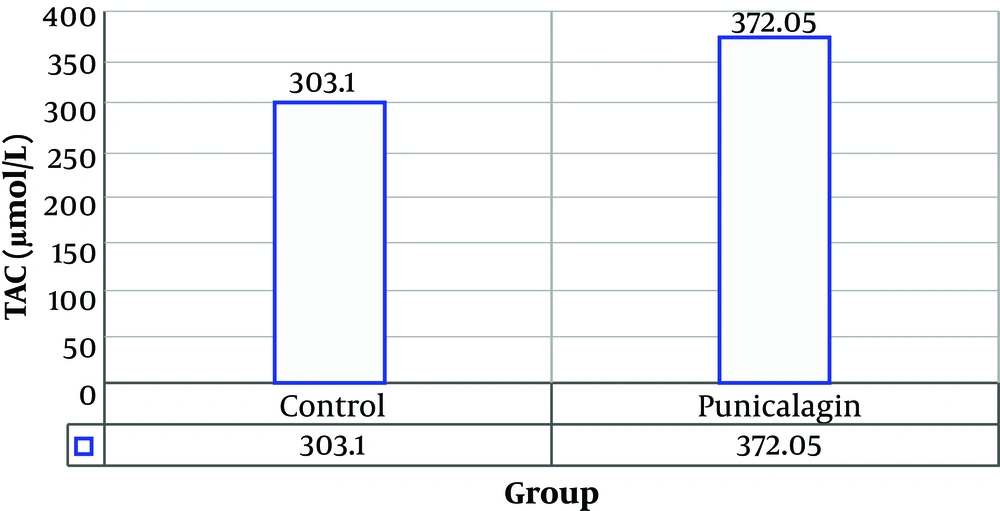
Figure 2 shows the total anti-oxidant capacity of different samples. The results indicate significant increase of tissue extract total anti-oxidant capacity on day seven in punicalagin nanofibrous-dressing treated group. The mean amount of TAC in tissue extract was 303.1 μmol/L and 372.05 μmol/L for the groups of control and treatment, respectively (Figure 2).
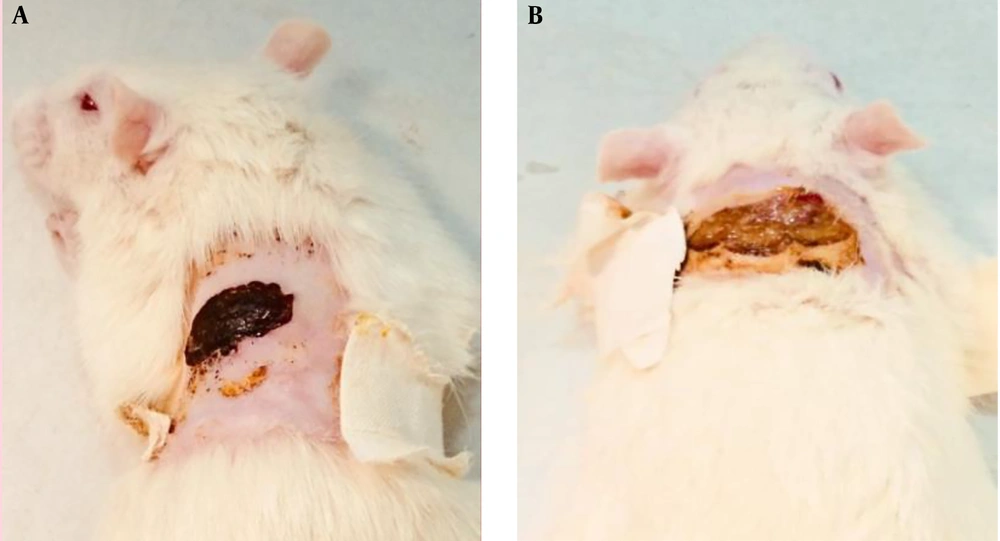
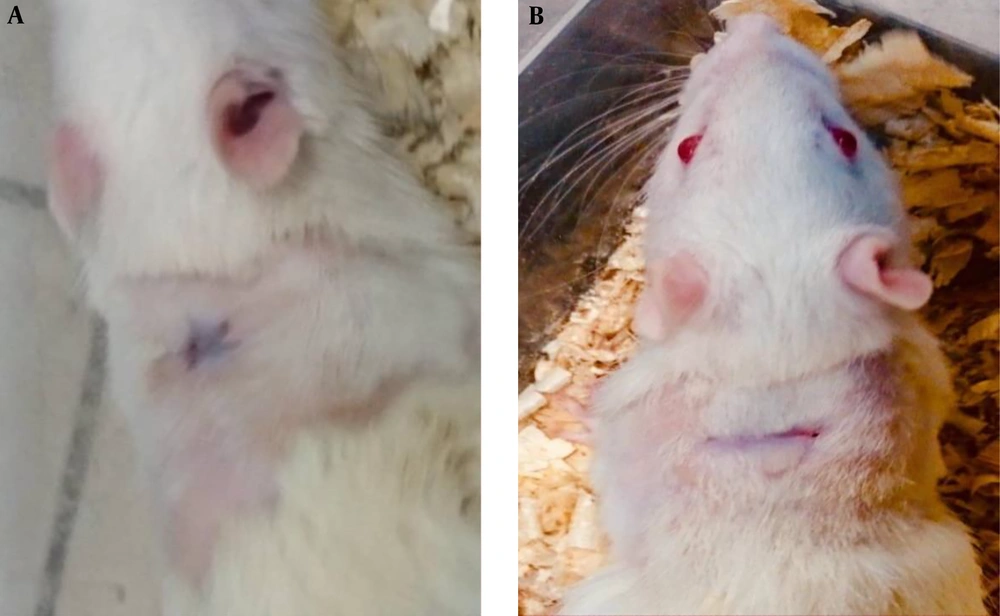
Figures 3 and 4 show the results of treatment of animals treated with punicalagin nanofibrous-dressing after seven and 21 days, respectively. A remarkable degree of wound healing was obvious in punicalagin nanofibrous dressing-treated animals as compared with the control group.
5. Discussion
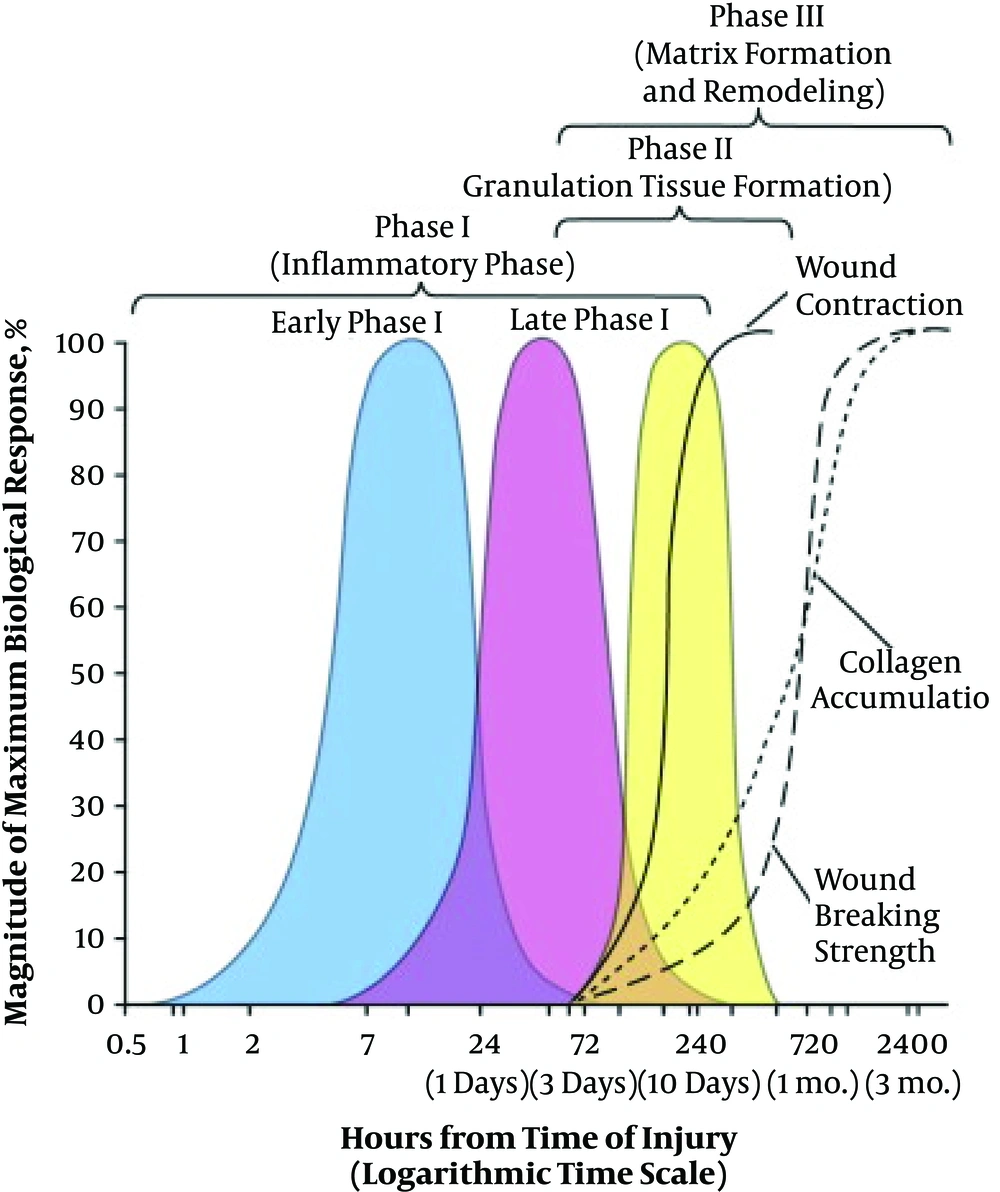
Complex processes of wound healing is divided to four phases, not precisely separated yet overlaped each other at some points: (1) hemostasis phase, (2) inflammatory phase, (3) proliferative phase, and (4) maturation and remodeling phase (Figure 5) (25).
Immediately after an injury, thrombi forms from the platelets, which are aggregated at first, at the injured vessel causing the pack of bleeding temporarily to stop hemorrhage. The inflammatory phase initiates simultaneously and chemotaxis leads to movement of different immune cells to the wound lesion secreting pro-inflammatory cytokines. A huge amount of reactive oxygen species (ROS), which are required to preserve the body from an infection are generated by inflammatory cells, remarkably neutrophils. In case of excessive production of ROS, neighbouring tissues can be injured (26).
In animals under specific conditions, for example diabetes, advanced age or immunosuppression, and retarded healing processes are reported, yet the exact mechanism is not clear yet. The balance between the levels of reactive oxygen species (ROS) and antioxidants defined the redox homeostasis, in which antioxidative enzymes have pivotal roles to scavenge ROS (26).
Hypoxia can impede wound healing as in different healing phases. Oxygen plays crucial roles, such as oxidative bacterial killing, collagen synthesis, angiogenesis, and epithelialization (27, 28). During energy production through the oxidative phosphorylation, oxygen is used and produced ROS contributes to oxidative injury (29, 30). The particular pathological role of ROS through the inflammatory phase is explained as neutrophils and macrophages secrete large amounts of ROS, pro-inflammatory cytokines (30), and proteolytic enzymes, such as matrix metalloproteinase (MMP) (31). In plasma membranes of inflammatory cells during phagocytosis, superoxide radical anions production emanates from NADPH oxidase (NOX2) expression and activation (32, 33). The produced ROS attack and kill the invading pathogens to help phagocytosis. However, excessive superoxide production can result in neighbouring tissues demolition. In the tuning of ROS, non-enzymatic antioxidants like low molecular weight compounds, such as glutathione, bilirubin, ubiquinone, vitamin C and E, carotenoids, phenolic compounds, and enzymatic antioxidants, including SOD, GPX, PRDX, catalase, are responsible (34).
Pomegranate (Punica granatum L.) is one of the ancient fruits, which is extensively used for it’s therapeutic properties. Interior network of pomegranate peels membranes contain considerable amounts of phenolic compounds, like hydrolysable tannins, such as punicalagin. Many researchers through various studies have reported that pomegranate peel could accelerate wound-healing process (21), yet wound-healing properties of purified punicalagins have not been studied.
5.1. Conclusions
Based on the increase of TAC, which we were detected in the punicalagin nanofibrous dressing treatment group at the inflammatory phase and the other anti-oxidant previously proved properties of Punica granatum L., the formulated nanofibers containing punicalagin, may be used as wound dressing.
According to the fact that the pomegranate extract has been utilized in folklore medicine for decades that reveals no toxic effects, using the presented skin dressing would be without hazard to animal and human health.