1. Background
The diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes mellitus (DM) that in addition to damaging the peripheral nervous system (PNS) has profound degenerative effects on central nervous system (CNS) referred to as diabetic encephalopathy (1). Cerebellum is one of the most important parts of CNS and is highly vulnerable to DM, which eventually leads to several clinical conditions. However, the underlining mechanisms of DM inducing such disturbances in cerebellum are not fully understood (2).
Mounting evidence demonstrates that endurance training (ET) is associated with multiple health benefits for patients with diabetes and ET profoundly improves glycemic control and blood lipid profiles (3). In addition to metabolic effects, ET has considerable effects on structure and function of nervous system, defined as neuroplasticity (4). Hence, ET may be a useful therapeutic intervention for neurodegeneration states such as DPN. Although some beneficial effects of ET on DPN state look like those of neural and metabolic adaptations, substantial part of its mechanism of action is elusive.
2. Objectives
The ADP-ribosylation factor 6 (Arf6) is a guanosine-5’-triphosphate (GTP)-binding protein with many functions such as actin cytoskeleton regulation, cell morphology and polarity, signal transduction, membrane traffic, endocytosis, and exocytosis (5). However, the roles of Arf6 in DPN are not explored yet. Therefore, to clarify this issue, the current study aimed at evaluating the effects of ET on gene expression of Arf6 in cerebellum of male Wistar rats with DPN.
3. Methods
The current study was performed according to health guidelines for welfare and use of animals in research at Animal Care Committee of the Medical University of Lorestan (LUNS.REC.1395. 170). Forty adult male Wistar rats were obtained from Pasteur Institute (Karaj, Iran) and housed four-per-cage in animal lab under standard conditions (12:12 hour light/dark cycle at room temperature of 20°C - 25°C) with access to food and water ad libitum. Before diabetes induction, animals were randomly divided into three groups: healthy control (C, N = 6), diabetic control (DC, N = 17), and diabetic trained (DT, N = 17).
Animals were kept in animal house for two weeks for acclimatization and reaching an optimum weight (at least 250 g) for diabetes induction (6). After an overnight fasting, diabetes induction was performed by a single intraperitoneal injection of streptozotocin (STZ) (45 mg/kg; Sigma, St. Louis, MO) solution, dissolved in a 0.5-mol/L of citrate buffered saline (pH 4.0). After two days, diabetes induction was checked by the measurement of tail vein blood glucose level using Accu-chek Compact Plus (Roche Diagnostics K.K., Tokyo, Japan). The animals demonstrating significant hyperglycemia (blood glucose levels more than 350 mg/dL) were selected as diabetic models and the ones without any trace of hyperglycemic were excluded from the study (in total, 14 rats were excluded from the diabetic groups). In addition, blood glucose levels were monitored every two weeks.
The treadmill training protocol was developed on previous protocols (7) consisting of six weeks of moderate-intensity (50% - 60% of maximal oxygen consumption) endurance aerobic exercise on a leveled motor-driven treadmill (model T510E, Diagnostic and Research, Taoyuan, Taiwan). The aerobic power of animals in terms of VO2max was obtained based on the relationship of VO2max to speed and treadmill slope (7). In the first week, the speed and duration of the treadmill running were 10 m/minute and 10 minute/day, respectively. The figures were then gradually increased until the 5th week, ending up with training speed and duration of 18 m/minute and 30 minute/day, respectively. To stabilize the obtained adaptations, training speed and duration were kept constant at the 6th week.
To model the DPN in rats, the procedures introduced by Calcutt were employed demonstrating that short-term diabetes induced by the STZ was the best considered as DPN models (8). In the current study, two weeks after confirmation of diabetes, behavioral nociception tests were assessed using Von Frey filaments and tail flick tests; the diabetic animals demonstrating thermal hyperalgesia and mechanical allodynia were considered as neuropathy models (9). To ensure perdurability of DPN, behavioral nociceptive tests were evaluated every two weeks, and the animals not exhibiting DPN symptoms were excluded from the study (in total, 12 rats were selected for DC and DT groups). Also, to control the acute effect of the last training session, behavioral nociception tests were assessed 48 hours after the last session of treadmill exercise.
Von Frey filaments test: To evaluate the mechanical allodynia, rats were placed in a transparent plastic box with a metallic grid floor. After 30 minutes of acclimatization to the environment, Von Frey filaments (Stoelting Co., Wood Dale, IL) with different stiffness ranging from 2 to 60 g were applied to plantar surface of the hind paw. The minimum stiffness of Von Frey filament, which induced the animal’s response through jumping, vocalization, shaking or flicking its paw, was recorded as paw withdrawal threshold (PWT).
Tail flick test: One hour before testing, rats were transferred to the behavioral tests section and gently placed in plastic restrainer. By a tail-flick instrument (LE-7406- Spain), radiant heat was focused on approximately 5 cm from the distal end of the rat’s tail and when the animal flicked its tail in response to the heat, the time was recorded as latency response. Cut off time of 20 seconds was used to prevent tissue damage. Each behavioral nociceptive test was performed three times with five-minute intervals. Final latency time was obtained by the average of the three latency responses.
Two days after the last exercise session in the 6th week of training, rats (N = 6 in each group) were anesthetized by inhalation of 2% halothane in a mixture of 20% O2, 80% CO2 (10), and their cerebellar tissue was removed under sterile conditions and immediately perfused in 4% paraformaldehyde (11).
Cerebellum samples (n = 6 in each group) were directly homogenized in 1 mL TRIzol (Invitrogen, Carlsbad, CA, USA) with a homogenizer. Total RNA was isolated from the cerebellum using QlAzol® lysis reagent (Germany, Qiagen) and chloroform (Germany, Qiagen) according to the manufacturer’s instructions. Briefly, about 50 mg of the cerebellum was separately homogenized in QlAzol® lysis reagent (1:10) and then centrifuged at 12,000 × g for 10 minutes at 4°C. After that, the product was mixed with chloroform (ratio 1:5) and centrifuged at 12,000 × g for 10 minutes at 4°C. Finally, after separating the water and mineral parts, RNA-containing part was mixed with isopropanol (1:5) and left at room temperature for 10 minutes. Then it was centrifuged at 12,000 × g at 4°C for 10 minutes. RNA-containing pellet was washed and diluted in RNase-free water. RNA concentration was quantitated by spectrophotometry (Eppendorf, Germany) and 260/280 nm ratios were 1.8 - 2, the desired purification. The cDNA synthesis was performed using Quanti Tect Reverse Transcription kit (Qiagen, Germany) based on the manufacturer’s instructions.
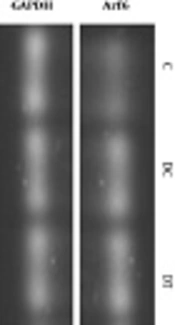
The real-time PCR experiments were conducted by the Premix SYBR Green II (Qiagen, Germany). Reaction mixture included 1 µL of cDNA, 1 µL of forward primer, 1 µL of reverse primer, 7 µL of DEPC water, and 10 µL of SYBR Green. All samples were measured in duplicate and primers were designed in accordance to Arf6 and GAPDH genes in gene bank of NCBI and nucleotides purchased from Qiagen (Germany). The real-time PCR was conducted with the following oligonucleotide primers: Arf6 [5’-AAAGGCATACATGGGGGGAGAT-3’ (forward) and 5’-GCGTTAGGATGCTCTGATGTGA-3’ (reverse)], and GAPDH [5’-AAGTTCAACGGCACAGTCAAGG-3’ (forward) and 5’-CATACTCAGCACCAGCATCACC-3’ (reverse)]. Thermal treatment involved the following cycles: 95°C for 10 minutes, 95°C for 15 seconds, 60°C for one minute (40 repetitions). The Arf6 gene was normalized to GAPDH levels and was calculated by 2-∆∆CT.
Statistical analyses were performed with SPSS version 19 (SPSS Inc., Chicago, IL, USA). Normality of data distribution was assessed by the Shapiro-Wilk test. Differences between groups were analyzed by means of ANOVA followed by LSD post hoc and P values of < 0.05 were considered statistically significant. The data were reported as mean ± standard error of the mean (SEM).
4. Results
4.1. Blood Glucose Levels
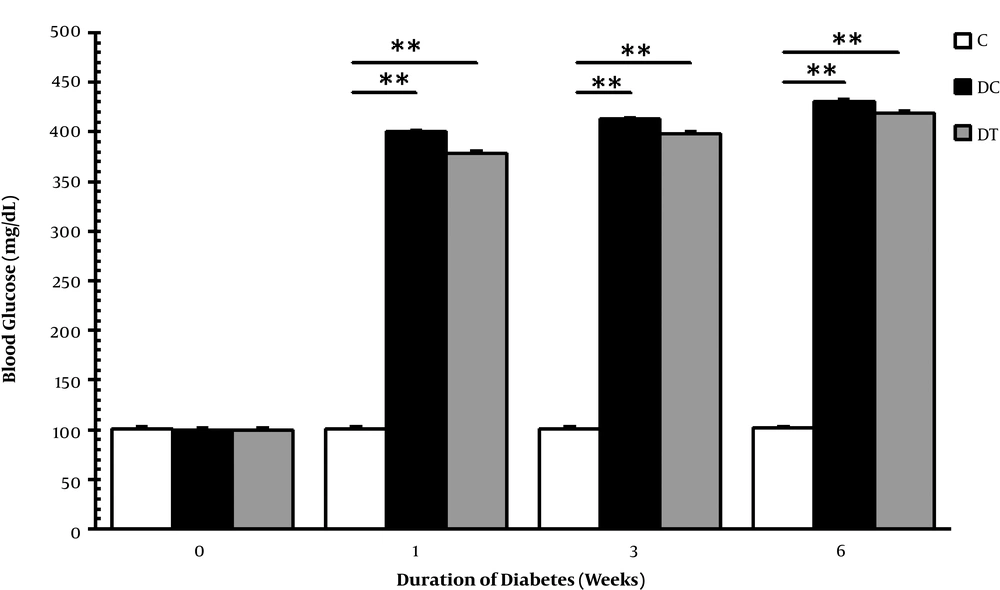
After a single dose of STZ injection into DC and DT animals, diabetes were induced successfully in most of the animals, and the ones that did not show hyperglycemia or died during the study were excluded and finally, six rats were selected for tissue extraction. Figure 1 shows the mean blood glucose levels of rats; as it can be observed, blood sugar levels of DC and DT groups were significantly higher than that of the group C in the 1st (P = 0.001, 0.001), 3rd (P = 0.001, 0.001) and 6th (P = 0.001, 0.001) weeks, although no significant difference was observed at baseline. The obtained results demonstrated that STZ successfully and profoundly induced diabetes in animals; therefore, hyperglycemia permanently remained throughout the study course and ET was not capable of damping these abnormalities.
Blood glucose levels in the C, DC, and DT experimental groups before STZ injection and one, three, and six weeks after diabetes induction. Comparing with the C, ** representing significant difference with the DC (P = 0.001) and the DT (P = 0.001) groups. This figure demonstrates that STZ injection successfully induced chronic hyperglycemia in rats.
4.2. Nociceptive Behaviors
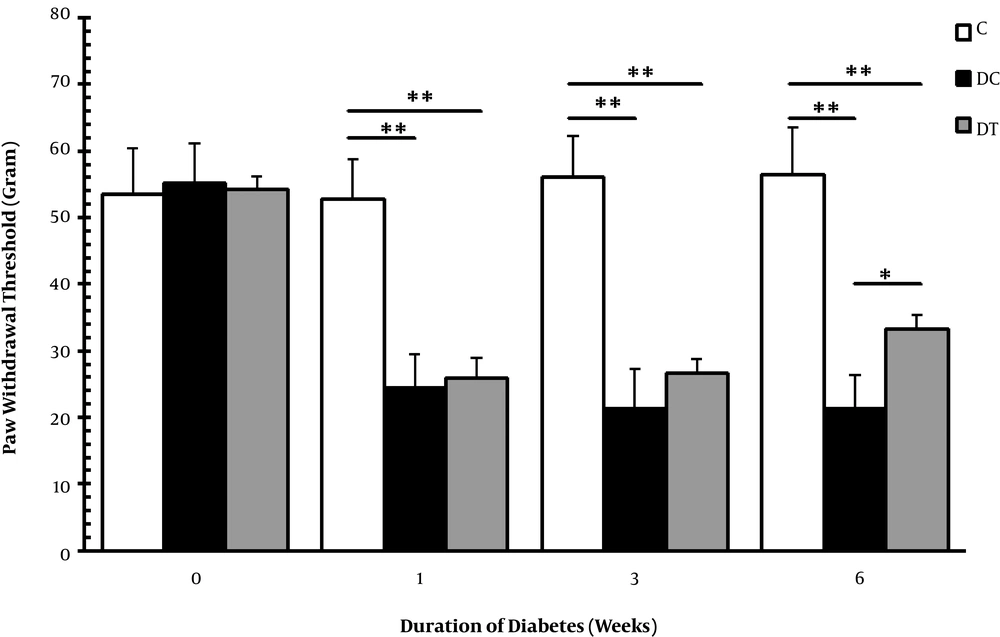
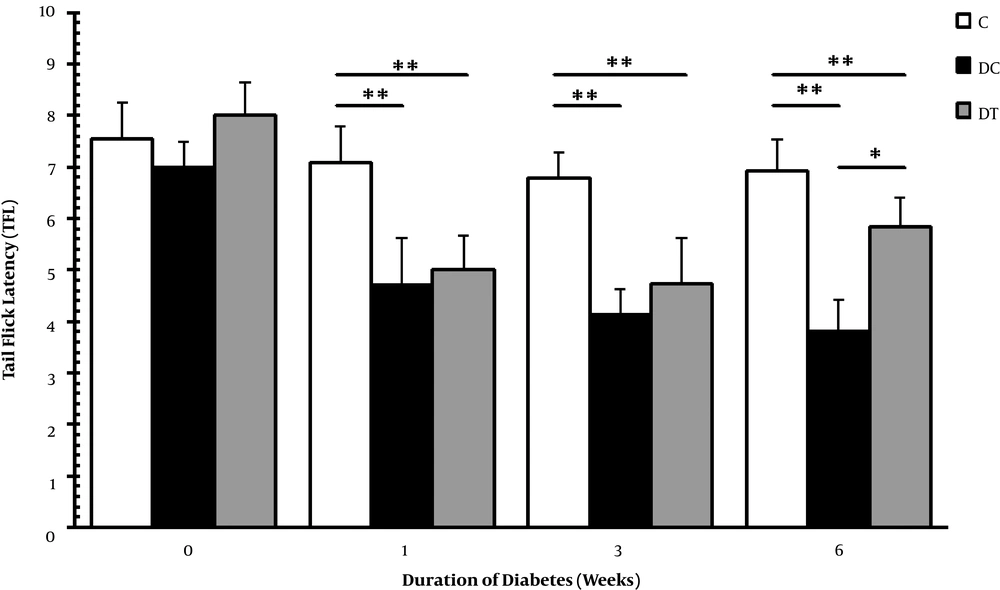
In the current study, thermal hyperalgesia and mechanical allodynia were assessed by Von Frey filament and tail flick tests to examine the nociceptive behaviors of diabetic animals. The diabetic animals with neuropathic pain in the DC and DT groups were selected, and the animals showing no nociceptive behaviors were excluded from these groups. Figures 2 and 3 show Von Frey filament and tail flick tests, respectively. According to these figures, mechanical allodynia and thermal hyperalgesia were evident in the DC and DT groups in comparison with the C group in the 1st (P = 0.001, 0.001), 3rd (P = 0.001, 0.001), and 6th (P = 0.001, 0.001) weeks. Moreover, these nociceptive behaviors persisted throughout the experiment with exception that in the 6th week, ET managed to damp mechanical allodynia in diabetic rats compared with the animals in the DC group (P = 0.017). It was insisted on using STZ diabetic neuropathy on the basis of previous report claiming that chronic pain shared global gene expression changes and some molecular pathobiology with neurodegeneration and also that it might be a functional examination of neural deterioration (12).
Mechanical allodynia in the C, DC, and DT experimental groups before STZ injection, and one, three, and six weeks after diabetes induction. Comparing with the C, ** representing significant differences with the DC (P = 0.001) and the DT (P = 0.001) groups. However, * demonstrates that mechanical allodynia was damped in the DT group compared with that of the DC group animals (P = 0.017), but there was still a significant difference with that of the C group (P = 0.001).
Thermal hyperalgesia in the C, DC, and DT experimental groups before STZ injection, and one, three, and six weeks after diabetes induction. Comparing with the C, ** representing significant differences with the DC (P = 0.001) and the DT (P = 0.001) groups. In addition, * demonstrates that ET could slightly affect thermal hyperalgesia in DPN rats of the DC and DT groups compared with that of the C group (P = 0.046).
4.3. The Arf6 mRNA in Cerebellum
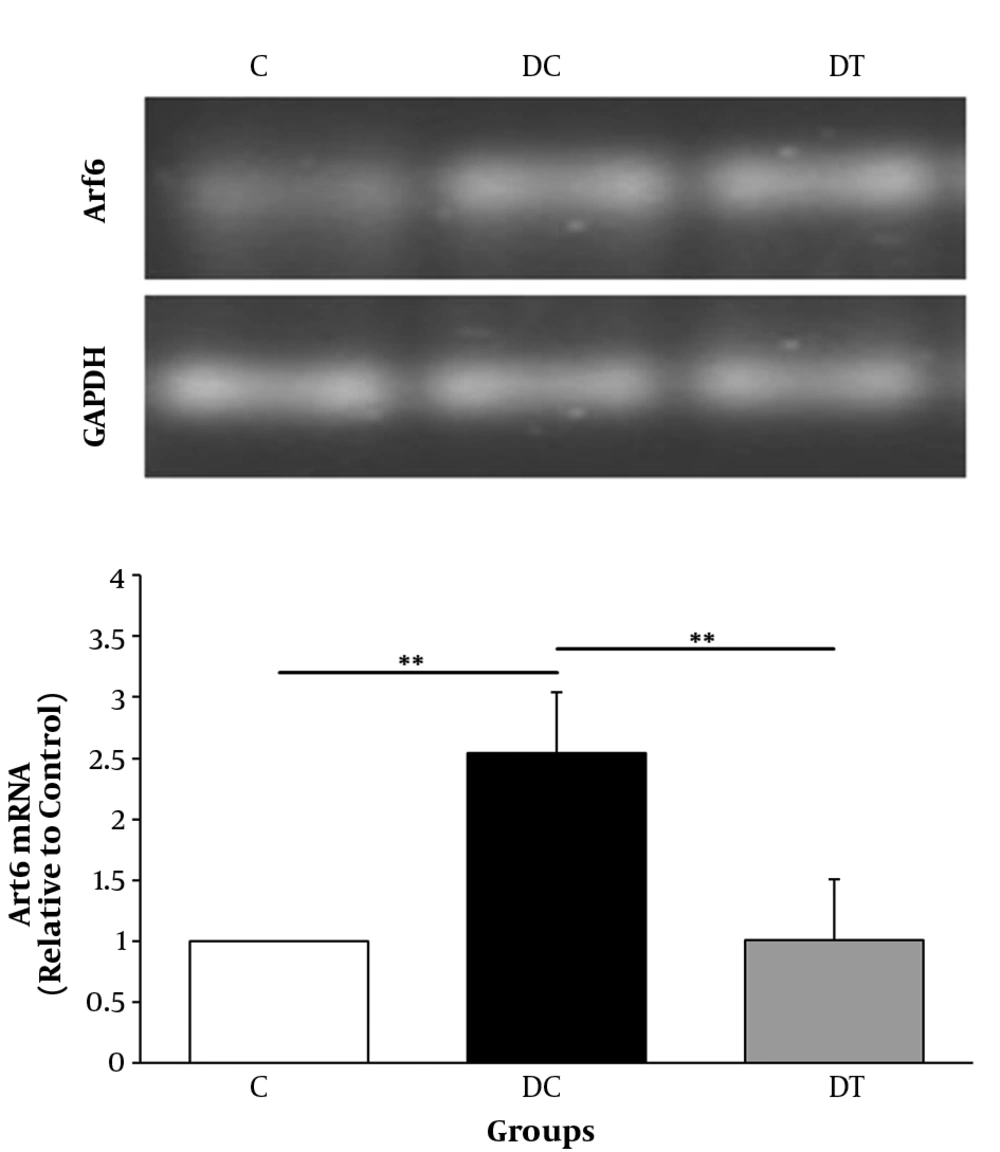
The mRNA levels of Arf6 in cerebellum of rats were measured after six weeks exposure to submaximal endurance training. In order to compare the levels of Arf6 gene expression in the C, DC, and DT groups, one-way ANOVA was used. Results showed a significant difference between groups; where the Arf6mRNA levels in the DC group were significantly higher than those of the C group (P = 0.003) indicating that DPN caused an elevated gene expression (2.5 fold changes) in cerebellum of animals. Also, chronic treadmill running in DPN rats was accompanied by a significant decrease of Arf6 gene expression, comparing with that of the DC (P = 0.004) groups indicating that ET profoundly damped the elevated Arf6mRNA levels in cerebellum of DPN rats. These data demonstrated that the gene expression of Arf6 in rat’s cerebellum was affected by ET and DPN state (Figure 4).
The Arf6 mRNA levels of the C, DC, and DT experimental groups. Comparing with the C group, ** shows that DPN state significantly increased the gene expression of Arf6 in cerebellum (P = 0.003). In addition, as compared with the DC groups, six weeks of ET exposure in the DT group could decrease this elevated gene expression (P = 0.003) mentioned by **. Also, the Arf6 mRNA of the DT group did not differ from that of the C group (P = 0.793).
5. Discussion
In the current study, for the first time Arf6 gene expression was evaluated in DPN state and it was observed that gene expression of this factor significantly increased in the cerebellum of rats with DPN. It was demonstrated that Arf6 played key roles in axonal outgrowth, dendritic branching, and spine formation in the cultured cortical and hippocampal neurons (13). However, there are a few in vivo examinations about the roles of this factor in neural disease. For example, it is demonstrated that Arf6 knockdown in Schwann cells inhibits migration of Schwan cells (14) and contributes to the axon myelination (15). On the basis of these findings, it appears that Arf6 is critical in CNS functions and its abnormal expression leads to numerous disorders; therefore, the elevated Arf6 gene expression in DPN state may be a compensatory response of CNS to attenuate the diabetic encephalopathy. Along with this assumption, Kumar et al., observed that DM up-regulates muscarinic receptors expression, gene expression of GLUT3, and insulin receptor mRNA in brainstem. They concluded that these expressional changes might be the characteristics of diabetic encephalopathy (15). Also, in the authors previous study, it was observed that in DPN state, the levels of KIF5B (a fast anterograde motor protein) mRNA significantly increased in the spinal cord and sciatic nerve (16). On the other hand, DPN was not always accompanied with compensatory responses and dependence on the examined factors could be related to down regulatory responses. For example, it is reported that the production of neurotrophic factors with supportive roles for neural tissue is reduced in DPN state (17).
Another finding of the current study was that ET decreased the expression of Arf6 in cerebellum of the DT group as compared with that of the DC group. Mounting evidence demonstrated that ET in DPN state could elicit beneficial plastic change in the CNS, which reverses multiple functional, structural, and behavioral abnormalities induced by DM (18-20). In line with these findings, the authors’ previous study showed that ET was able to damp the increased levels of the KIF5B of spinal cord and sciatic nerve in DPN state; therefore, it was concluded that DPN state affected transcriptional and posttranscriptional neural machinery system and ET was an efficient tool to adjust this abnormality (16). It was also observed in the current study that ET was capable of damping DPN-induced neural changes of Arf6 expression without changing the blood glucose levels. Although the exact mechanisms of these adaptations are not clear, presumptive mechanisms for this exercise-induced neuroplasticity in DPN state could be insulin sensitivity (21), elevation of neurotrophic factors, and insulin-like growth factor (IGF-1) production (22).
The current study observations also included the lack of normal blood sugar (Figure 1), mechanical allodynia (Figure 2), and thermal hyperalgesia (Figure 3) in response to ET with the exception that after six weeks of training, nociception was attenuated, but not eliminated in DT group. Generally, it is impossible for STZ diabetic rats to show hypoglycemia and significant decrease of glucose levels only through ET. The current study results revealed that nociception could not be protected by exercise alone. Although the authors’ previous study showed that four weeks of treadmill running could not damp thermal hyperalgesia in tail flick test (23), several papers reported that ET delayed the onset of diabetic pain (24) and also reduced tactile hypersensitivity (25), mechanical allodynia (26, 27), and hyperalgesia (28). These conflicts could be due to different models, intensity, and duration of exercise and/or the different painful stimuli (28).
5.1. Conclusions
The current study showed that cerebellar Arf6 gene expression increased in DPN state. ET, as a non-pharmacologic therapeutic intervention, could damp this elevation in diabetic rats. It seems that Arf6 is an important mediator of stress-induced plasticity in the nervous system, but the exact physiologic roles of Arf6 in DPN state are not clear. Hence, identifying the importance of Arf6 in neurodegenerative diseases such as DPN state needs further studies.