1. Background
The inflammation usually occurs in response to injurious stimuli, including physical injury, trauma, infectious microorganisms, toxic chemical substances, ischemia, or tumor growth (1, 2). Some characteristics of inflammatory responses are comprised of pain, swelling, heat, redness, edema, and loss of function (3). The inflammation consists of different phases, including the release of chemotactic factors, increased blood circulation, and increased capillary permeability, allowing for cellular infiltration, followed by either an acute resolution of tissue damage or persistence of the response that might contribute to fibrosis or dysfunction of the tissues and organs (4). Although inflammation is a defense mechanism, it could be disadvantageous if the stimulus insists for long-term courses as it may cause painful inflammatory problems, Including gastritis, arthritis, etc. (5). The inflammation is generally related to pain as a secondary mechanism, caused by secretion of analgesic mediators (6). Pain as an unsightly feeling can be either acute or chronic and it is an outcome of involved neurochemical mechanisms in the nervous system (7). In addition, pain is the most common reason for seeking medical and pharmaceutical care and it is the most prevalent sign of various pathologies and imposes a high expenditure of pharmaceutical and health burden on the society (8).
Although the inflammation and pain are commonly remedied by nonsteroidal anti-inflammatory drugs (NSAIDs), their long-term use is closely associated with serious toxic effects, including gastrointestinal ulcers, renal disorders, hepatic abnormalities, and metabolic disturbances (9-11). In addition, opioids (narcotics), including natural (derived from the opium poppy plant) and synthetic narcotics are potent drugs in reducing the swelling and pain. Opioid drugs have diverse psychological and physical side effects such as gastrointestinal bleeding, nausea and vomiting, cognitive impairment, respiratory depression, hyperalgesia, endocrine-hypogonadism, tolerance, withdrawal, and addiction (12-17). Therefore, there are efforts to find safe and new anti-inflammatory and analgesic medications with the least side effects. Traditional medicine and medicinal plants are extensively used in reducing the swelling and pain symptoms (18, 19).
Propolis or bee glue is a gummy resinous material that honey bees amass it from plant exudates to make seal holes in the beehive (20, 21). Because of waxy and supple quiddity of propolis, honey bees exploit it in the making and renovation of their beehive (22, 23). In addition, many studies have shown that there are about 300 compounds in propolis, including resin (50%), wax (30%), essential oils (10%), pollen (5%), and other organic compounds (5%) (24, 25). Propolis has useful effects on body health and it has been extensively used in folk medicine to treat many illnesses for many years. The Greek and the Roman physicians also acknowledged the potential of propolis by employing it in wound treatment, as an antiseptic and cicatrizing agent, and as mouth disinfectant. The Persians used propolis in eczemas, myalgia, and rheumatism remedy. The Incas also described propolis as an antipyretic drug (26, 27). In the past few years, numerous literatures have been attributed to propolis activities such as anti-inflammatory, anti-tumoral, antiviral, antibacterial, anti-fungal, and antinociceptive (24-28). Propolis activates immune cells that produce cytokines (28). It is also used to treat muscle and articulation inflammations, infections, rheumatisms, and torsions (29).
2. Objectives
This research aimed to test the effects of propolis on inflammatory and nociceptive models in male mice and rats.
3. Methods
This experimental study was performed in 2018 at Payame Noor University, Kermanshah, Iran. This study was approved by the Research Ethics Committee of Payame Noor University (code: IR.PNU.REC.1397.080) and carried out according to the ethical guidelines for experimental investigation in animals.
Preparation of ethanolic extract of propolis: Approximately 100 g propolis was obtained from bees’ hives in Ardebil province, Iran and stored at 4°C. After dehydration, the dried samples were ground to make a fine powder. Ethanolic extract of propolis (EEP) was prepared by adding 2 g of powder to 25 mL of 10% - 95% ethanol in tubes and shaking at 70°C for 30 minutes. In the next step, the extract was centrifuged to acquire the supernatant (30).
3.1. Animals
Animals used in this study were comprised of 90 male BALB/c mice (25 - 30 g) and 30 male Wister rats (250 - 300 g). They were maintained at a humidity of 50 ± 5%, a temperature of 22 ± 1°C, with a 12 hours light/dark cycle, and given ad libitum access to food and water.
3.2. Anti-Inflammatory Study
3.2.1. Xylene-Induced Ear Edema
For acute inflammation assessment, 30 mice were randomly assigned to five groups of 6 animals, including EEP (100, 200, and 400 mg/kg), positive control (dexamethasone, 15 mg/kg) or normal saline (the control group). Dexamethasone, commonly used as positive control anti-inflammatory agent, showed potent anti-inflammatory effects on xylene-induced ear edema. Sixty minutes after the intraperitoneal injections, 0.03 mL xylene was injected into the anterior surface of the right ear, while the left ear defined as the control. Two hours after the xylene injection, mice were deeply anesthetized and ears were removed. Circular sections were taken with a diameter of 7 mm and carefully weighed. An increase in the weight of the right ear punch compared with the left ear punch was indicated the edema (31).
3.2.2. Cotton Pellet Granuloma
The chronic anti-inflammatory test carried out using cotton pellet granuloma model. Rats were randomly assigned to five groups (n = 6), including distilled water (control), indomethacin (10 mg/kg, the positive control), and 100, 200, and 400 mg/kg of EEP. Indomethacin, the positive control used in the study, which is known as an anti-inflammatory agent in cotton pellet granuloma model (32, 33). The animals were anesthetized with ketamine (100 mg/kg). In brief, after back skin disinfection with 70% ethanol and shave, a longitudinal incision of the skin was made in the lumbar region. Subcutaneous tunnels were created by blunted forceps and a sterilized, pre-weighed cotton pellet (15 ± 1 mg) was placed on both sides in the scapular region. Thirty minutes before the test, the animals were treated with distilled water, indomethacin, or extracts were orally-administered daily for 7 days. Then the animals were sacrificed in the 8th day, the pellets were dissected out, and dried in an oven at 60°C until the weight stabilized. Then the net dry weights and the percent inhibition increase in the weight of the cotton pellets were determined (32, 33).
3.3. Antinociception Study
3.3.1. Formalin Test
Thirty mice were randomly assigned to five groups of 6 animals. In this test, 45 minutes prior to formalin test, normal saline as the control, morphine (10 mg/kg) as the positive control or 100, 200 or 400 mg/kg of EEP were administered intraperitoneally. Morphine, commonly used as the positive control antinociceptive agent, showed potent analgesic effects on formalin test (34, 35). In this test, mice placed in a transparent enclosure, then 20 µL of 2.5% formalin was injected into the right posterior paw. The formalin-induced paw licking response was designed as representative of the nociceptive behavior. After formalin injection, 0 - 5 and 20 - 30 minutes were recorded as the total time spent in licking and biting the injected paw (34, 35).
3.3.2. Acetic Acid-Induced Writhing
Thirty mice were randomly assigned to five groups of 6 animals. Forty-five minutes before the peritoneal irritation, mice were treated with EEP, 0.9% normal saline (control) and indomethacin (10 mg/kg) by oral administration. Then the animals were injected intraperitoneally with 1% acetic acid (0.1 mL/10g body weight). The writhing results were recorded after 10 minutes of acetic acid injection and counted for 10 minutes (34-37). Antinociceptive activity was distinguished by a decrease in the average of writhing numbers in the treatment groups compared with the control group, and it was calculated as %inhibition of abdominal constrictions using the following formula (37): [mean of (control - test group)/control group × 100%].
3.4. Data Analysis
The results were described as mean ± SEM and differences among groups were statistically excavated by one-way analysis of variance (ANOVA) followed by the Tukey method as a post hoc test. The significance level was set at P < 0.05.
4. Results
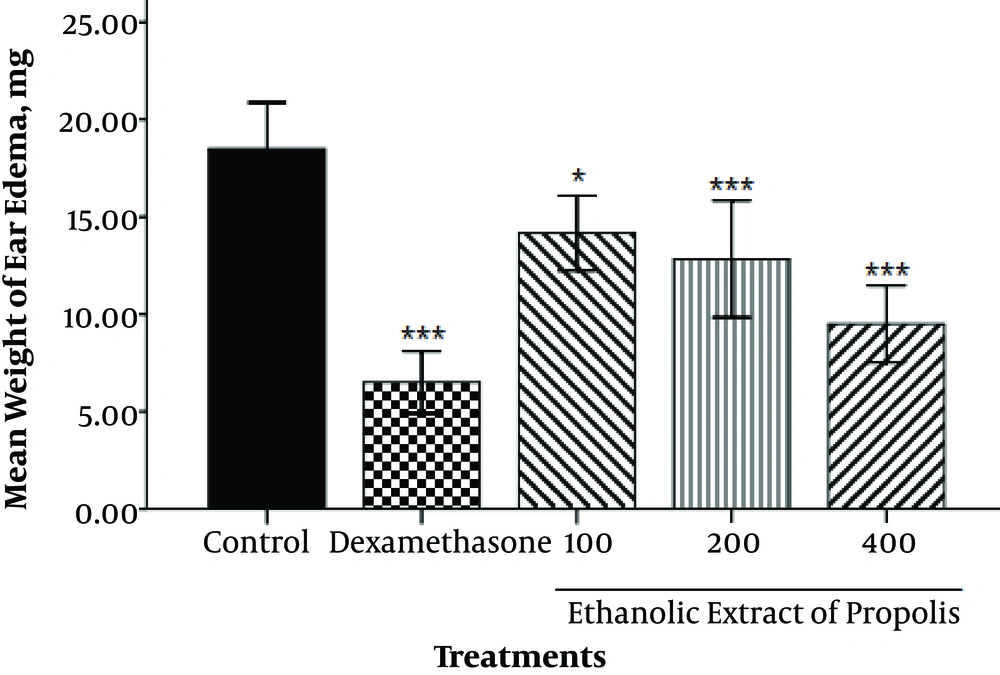
4.1. Xylene-Induced Ear Edema
The mean weight of ear edema in the control, dexamethasone, 100, 200 and 400 mg/kg of EEP groups was determined as 18.5 ± 0.92, 6.50 ± 0.62, 14.17 ± 0.75, 12.83 ± 1.17 and 9.50 ± 0.76 mg, respectively. As shown in Figure 1, a significant decrease was observed in weight of ear edema in the dexamethasone (P < 0.001), and EEP at 100 (P < 0.05), 200 (P < 0.001), and 400 mg/kg (P < 0.001) in comparison to the control group.
4.2. Cotton Pellet Granuloma
As shown in Table 1, a significant decrease was observed in granuloma weight in the Indomethacin (P < 0.001) and EEP (200 and 400 mg/kg, P < 0.001), while EEP insignificantly decreased the granuloma weight at 100 mg/kg. At 100, 200, and 400 mg/kg of EEP, the percent reduction of granuloma weight was 10.05%, 19.15%, and 17.53%, respectively, as compared to the control group, whereas the standard drug indomethacin (10 mg/kg) showed a reduction of 44.15%.
| Treatments | N | Granuloma wt., mg | Inhibition, % |
|---|---|---|---|
| Control | 6 | 51.33 ± 1.74 | - |
| Indomethacin | 6 | 28.67 ± 1.78*** | 44.15 |
| EEP100 | 6 | 46.17 ± 0.79 | 10.05 |
| EEP200 | 6 | 41.50 ± 0.99*** | 19.15 |
| EEP400 | 6 | 42.33 ± 0.80*** | 17.53 |
4.3. Formalin Test
Results of the antinociceptive effects of EEP on pain induced by formalin in mice are shown in Table 2. Both phases of formalin-induced nociception were significantly inhibited in mice pretreated intraperitoneally with morphine and EEP. In the first phase (0 - 5 minutes), the inhibitions were 88.97% (P < 0.001), 14.11% (P < 0.01), 26.66% (P < 0.001), and 62.31% (P < 0.001) in morphine and EEP at 100, 200, and 400 mg/kg, respectively. In the second phase (20 - 30 minutes), the inhibitions were 89.85% (P < 0.001), 25.73% (P < 0.001), 46.40% (P < 0.001), and 56.32% (P < 0.001) in morphine and EEP at 100, 200, and 400 mg/kg, respectively.
| Treatments | N | First Phase (0 - 5 min) | Inhibition, % | Second Phase (20 - 30 min) | Inhibition, % |
|---|---|---|---|---|---|
| Control | 6 | 65.00 ± 1.93 | - | 113.33 ± 2.32 | - |
| Morphine | 6 | 7.17 ± 0.31*** | 88.97 | 11.50 ± 1.02*** | 89.85 |
| EEP100 | 6 | 55.83 ± 0.94** | 14.11 | 84.17 ± 2.34*** | 25.73 |
| EEP200 | 6 | 47.67 ± 1.47*** | 26.66 | 59.67 ± 4.54*** | 46.40 |
| EEP400 | 6 | 24.50 ± 2.08*** | 62.31 | 49.50 ± 5.91*** | 56.32 |
4.4. Acetic Acid-Induced Writhing
As shown in Table 3, a significant decrease was observed in the number of writhes in the indomethacin (P < 0.001) and EEP at 100 (P < 0.05), 200 (P < 0.001), and 400 (P < 0.001) mg/kg. The inhibition percentage of the number of writhes with indomethacin and EEP at 100, 200, and 400 mg/kg are 78.96%, 21.73%, 38.96%, and 40.68%, respectively.
| Treatments | N | Number of Writhes | Inhibition, % |
|---|---|---|---|
| Control | 6 | 48.33 ± 1.14 | - |
| Indomethacin | 6 | 10.17 ± 1.30*** | 78.96 |
| EEP100 | 6 | 37.83 ± 2.77* | 21.73 |
| EEP200 | 6 | 29.50 ± 2.32*** | 38.96 |
| EEP400 | 6 | 28.67 ± 2.60*** | 40.68 |
5. Discussion
In the present investigation, the administration of EEP showed potent anti-inflammatory effects in 2 models of inflammation, including xylene-induced ear edema and cotton pellet granuloma tests. In addition, administration of EEP showed potent antinociceptive effects in 2 models of pain, including formalin test and acetic acid-induced writhing test.
This is the first study conducted on anti-inflammatory and antinociceptive properties of Iranian propolis extracts. The present finding is supported by a previous study about antinociceptive and anti-inflammatory properties of hydroalcoholic extract of Brazilian red propolis (24). Xylene-induced ear edema is a simple and reliable model of acute inflammation for evaluating potential anti-inflammatory agents (38). This model of inflammation presumably is initiated by the release of histamine, kinin, fibrinolysin, and phospholipase A2. These inflammatory intermediaries induce edema by vasodilation and increased vascular permeability (38-40). In this model, EEP was able to reduce acute inflammation in a dose-dependent manner. These results suggest that EEP may interfere with the actions of inflammatory mediators and produce anti-inflammatory effects. The cotton pellet granuloma is a convenient model for evaluating chronic inflammation. This type of inflammation is characterized by the proliferation of macrophages and fibroblasts as well as granulocyte infiltration (33, 41). The inhibitory effects of EEP may be due to the decrease of mentioned agents. The decrease in granuloma weight indicates that the anti-inflammatory activity of EEP was not in a dose-dependent manner. The inhibition percentage of granuloma weight produced by 200 mg/kg dose of EEP was significantly higher than that produced by the other two doses (100 and 400 mg/kg).
In this study, the antinociceptive activity of EEP was assessed using the acetic acid-induced writhing test and formalin test in mice. The writhing method induced by acetic acid is commonly described as a peripheral type of antinociceptive assessment of medicines (38, 42). The peripheral pain is initiated by the release of intermediaries such as bradykinin, lipoxygenases, substance P, prostaglandins and cyclooxygenases, as well as some cytokines such as interleukin-1 (IL-1), interleukin-8 (IL-8) and tumor necrosis factor (TNF) (38, 43). Formalin test is a valid model in analgesic studies that consists of 2 stages. The first stage (0 - 5 minutes) is characterized by neurogenic pain caused by direct stimulation of nociceptors. Substance P and bradykinin are thought to participate in this phase. The second stage (20 - 30 minutes) is specified by inflammatory pain, an action in which some inflammatory intermediaries are imagined to be involved, including histamine, prostaglandins, serotonin, and bradykinin. In fact, centrally acting medicines prevent both stages equally, while peripherally acting medicines prevent the second stage (38, 40, 44). In this study, EEP relieved the pain in 2 stages in a dose-dependent manner. The results obtained from the formalin test were in agreement with the results from the writhing test, indicating that the extract had central and peripheral antinociceptive activities. The results obtained from inflammation and pain animal methods confirm that EEP may have the ability to reduce the production of inflammatory and pain response mediators.
It is known that phytochemicals such as flavonoids, phenolics, terpenoids, etc. have antinociceptive, anti-inflammatory, and antioxidant activities (45-48). Over 500 compounds such as flavonoids, phenolics, phenylpropanoids, terpenoids, stilbenes, lignans, coumarins, and their prenylated derivatives have been identified in propolis from many countries up to 2012 (49). Flavonoids have been widely shown to prevent the production of prostaglandins, arachidonic acid, histamine, bradykinins, etc., which participate in the inflammation and pain (50, 51). The major constituents of propolis, flavonoids, generally participated in pharmacological processes of Propolis. From 2000 to 2012, 112 flavonoids were identified in propolis. According to the chemical structure, flavonoids in propolis are arranged into flavones, flavonols, flavanones, flavanonols, chalcones, dihydrochalcones, isoflavones, isodihydroflavones, flavans, isoflavones, and neoflavonoids. In addition, flavonoid glycosides were identified that were very rare in propolis. They are isorhamnetin-3-O-rutinoside and flavone C-glycoside (49). Some studies previously described anti-inflammatory and antinociceptive activities of flavonoids. For instance, Chalcones have been introduced as selective cyclooxygenase-2 inhibitors (52). Also, isoflavone isolated from Polygala molluginifolia had an antinociceptive effect on mice (53).
Propolis is rich in phenolics, including cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid, and their derivatives (52) that all of them were reported to possess anti-inflammatory and antinociceptive activities (54-57). Terpenoids isolated from propolis consist of types of monoterpenes and sesquiterpenes (49) that previous studies have shown the antinociceptive and anti-inflammatory activities of such compounds (58). Altogether, it can be concluded that ethanolic extract of propolis has potential anti-inflammatory activity against both acute (xylene-induced ear edema) and chronic inflammation (cotton pellet induced granuloma). The extract also shows antinociceptive activity, mediated both centrally (formalin test) and peripherally (acid-induced writing test and formalin test). Therefore, it can be concluded that some chemical compounds in propolis may be responsible for the antinociceptive and anti-inflammatory activities.