1. Context
1.1. Ionizing Radiation/Non-Ionizing Radiation and Their Biological Effects
Today, modern lifestyle interconnects human populations with electromagnetic and electric devices such as mobile phones, radar satellites, wireless systems, telecommunication cables, etc.
People are persistently exposed to radiations emitted by commercial and local electromagnetic devices. The radiations known as electromagnetic waves have frequencies in the range of 3 kHz to 300 GHz with high wavelengths about 1 mm.
Radio waves and microwaves have low amplitude with the range of 800 - 2200 MHz. In other words, these are non-ionizing waves with no the capability of breaking the chemical bonds in biomolecules. On the other hand, waves such as Gamma and X-rays, known as ionizing waves, with short wavelengths and very high frequencies could break chemical bonds in biological molecules and subsequently cause cellular and tissue damage. Other ionizing radiations include excessive exposure to solar radiations and cosmic rays. Many people are constantly exposed to ionizing radiations due to occupational or medical reasons.
In recent years, biological impacts of electromagnetic waves emitted from mobile phones, Wi-Fi devices, and magnetic resonance imaging scans on human organs are assessed by a standardized unit called specific absorption rate (SAR), which is equivalent to the absorption of energy emitted from this device by body tissue. The SAR is calculated based on the following equation watts/kilogram (1):
SAR = σE2/ρ
σ = mean electrical conductivity of tissue (sec/m)
ρ = mass density of tissue (kg/m3)
E= electrical field strength
The adverse effects of exposure to ionizing and non-ionizing radiations are greatly debated. The adverse effects of exposure to ionizing and non-ionizing radiations occur by thermal and non-thermal effects. High frequency radiations with heating properties increase the tissue temperature; the effect is known as thermal effects.
It is proven that radiations emitted by microwaves can cause adverse thermal effects on organs and functions of the human body. The damage induced by thermal effect might be due to tissue inability to dissipate excessive heat. On the other hand, tissue with more blood vessels and more perfusion networks are less susceptible to damages (2).The effects depend on several factors such as frequency, time of exposure, the metabolic activity of the tissue, the tissue water content, and the perfusion network of the tissue (3). It is recently reported that SAR values greater than 4 W/kg could increase the tissue temperature up to 1°C (4).
Another damaging effect caused by radiation is the non-thermal effect indicated by impaired functions of the heart (5), eye (6), liver and kidney (7), encephalon and cerebellum (8), blood cells (9), nervous system (10), endocrine system (11), and reproductive system (12, 13) in humans and animals; it is greatly documented in the literature. The non-thermal biological effects of ionizing and non-ionizing radiations mainly depend on the intensity, frequency, and exposure duration.
1.2. Ionizing /Non-Ionizing Radiation and Cell Function
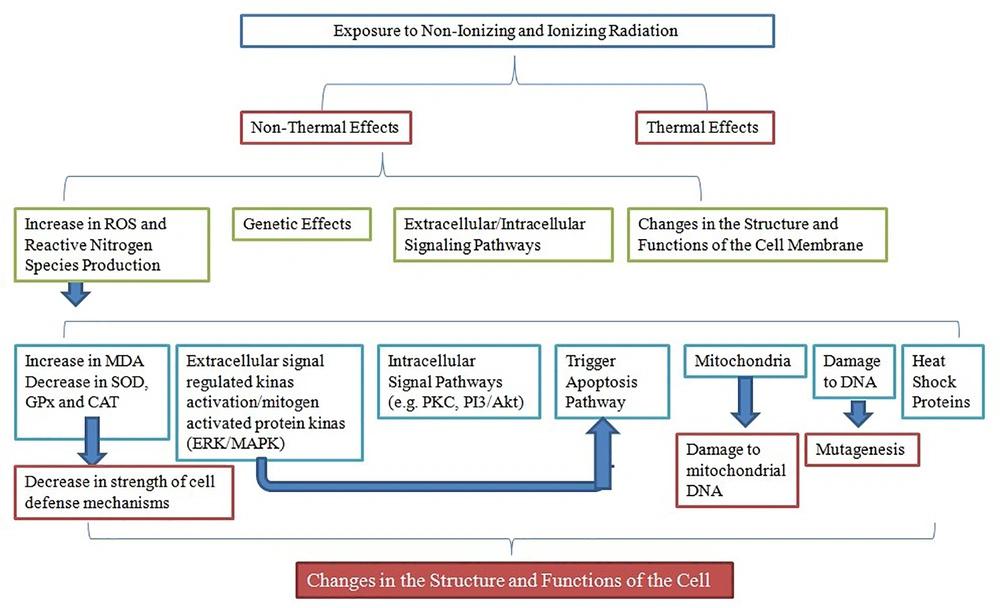
In the last few decades, ionizing and non-ionizing radiations in the etiology of cellular functions and disorders are highlighted. Numerous studies reported that ionizing and non-ionizing radiations affect cell metabolism, cell differentiation (14), apoptosis pathway (15), heat shock proteins and mitochondria (16). Some reports demonstrated that acute exposure to non-ionizing radiation could have direct impact on neural function in humans (17) and neuroblastoma cells (18). In addition, recent studies suggest that exposure to radio waves by the release of ROS, induce extracellular-signal-regulated kinase activation of the epidermal growth factor receptor (EGFR) (19).
Previous investigations indicated that radiations can induce several damages at DNA level (20), changes in the structure and functions of plasma membrane [i.e., NADH (nicotinamide adenine dinucleotide) oxidase and phosphatidylserine] (21), Ca2+ signaling and NMDA (N-methyl-d-aspartic acid) receptor functions (22), voltage-gated calcium channels (23), and oxidative stress (10). These changes could cause cellular and organ dysfunctions.
2. Methods
2.1. Ionizing/Non-Ionizing Radiation, Oxidative Stress, and the Concept of Adaptive Response
The current review study focused on the effects of oxidative stress after exposure to ionizing and non-ionizing radiations on cell function of antioxidant defense.
2.1.1. Oxidative Stress
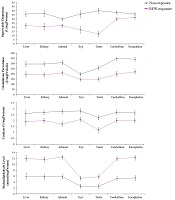
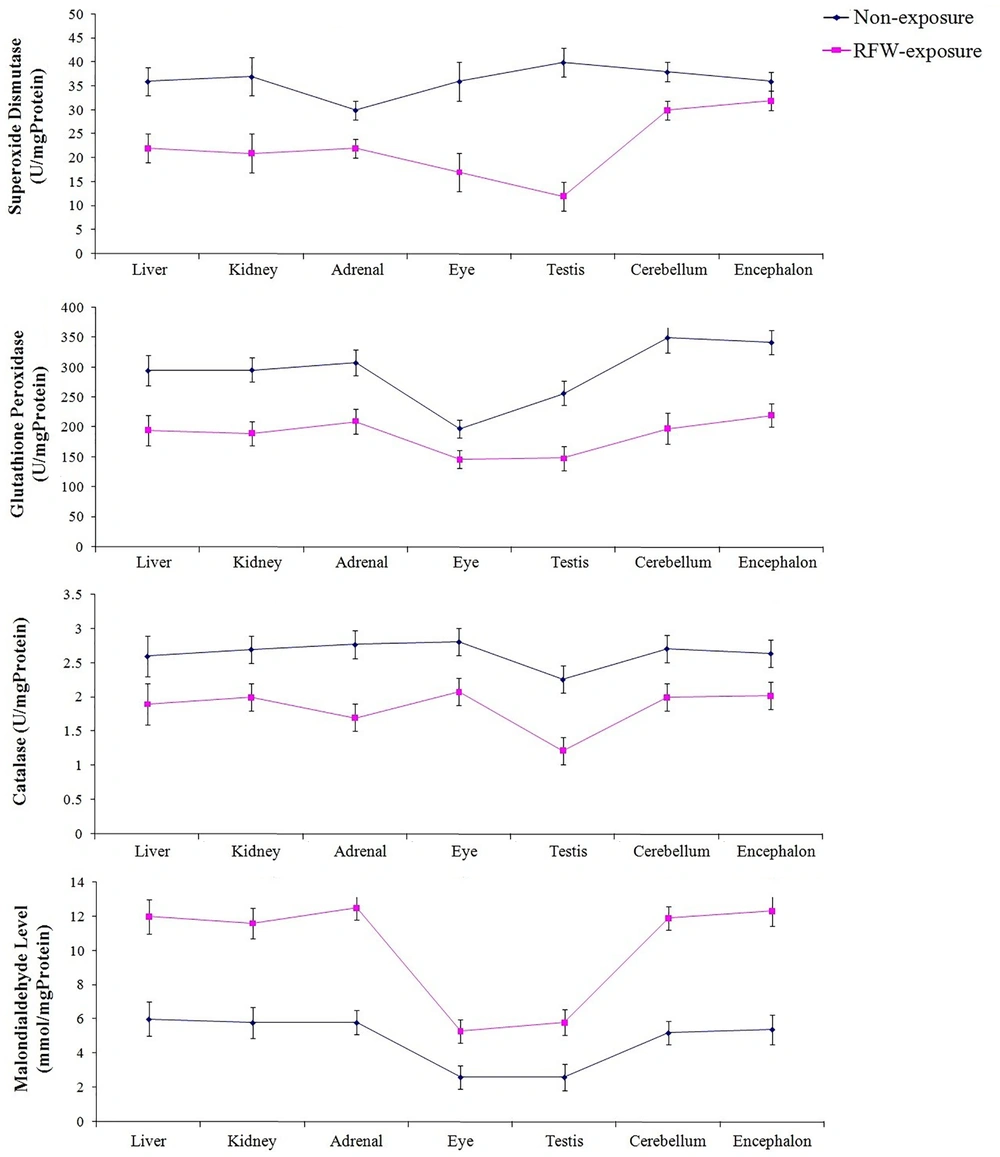
Oxidative stress refers to overproduction of reactive oxygen species (ROS) and /or deficiency in antioxidant defense mechanisms. This imbalance can be due to environmental stimuli such as acquaintance to ionizing radiation (e.g., Gamma radiation, cosmic rays, and medical X-rays) or non-ionizing radiations such as radiofrequency waves emitted by mobile phones, wireless and other electrical devices. Several animal and human studies indicated that exposure to ionizing and non-ionizing radiations could induce oxidative damage to proteins, lipids, and DNA (10, 24). It is believed that exposure to ionizing radiations can change the atomic structure of biomolecules through oxidizing reactions either via direct interactions of radiation with target macromolecules (i.e., DNA) or through water radiolysis-induced products (24), while, non-ionizing radiations could cause different cellular dysfunction. In several previous studies, it was shown that exposure to 900 MHz radiofrequency wave emitted by base transceiver station can change oxidative status in various tissue of laboratory animal models (Figure 1) (6, 7, 9).
A part of molecular oxygen in electron transport chain of mitochondria reduces and generates ROS under physiological conditions (10). ROS at high concentrations induces pathological impacts via reaction with major macromolecules such as lipids, proteins, and nucleic acids which leads to oxidative damage and cell injury. However, low or transient levels of ROS have physiological roles via cellular proliferation and survival signaling pathways such as protein kinase C, phosphoinositide 3-kinase/Akt8 virus oncogene cellular homolog, extracellular signal-regulated kinase/mitogen-activated protein kinase pathways and nitric oxide signaling (19, 25). Protein kinase C, via H2O2 activation, plays a role in cell signaling pathways (25) (Figure 2).
2.1.2. The Concept of Adaptive Response
It seems that oxidative damage or ROS levels generated after exposure to ionizing and non-ionizing radiations have beneficial biological effects.
However, there is much doubt regarding the possible risks of low-dose radiation on humans. There are several reports that low-dose radiations have no deleterious effects on biological systems or some even reported beneficial effects (26, 27). It is well known that radiotherapy is one of the standard treatments for progressive stage of cancer. A sophisticated equilibrium of maximum dose exposure to malignant tumor and minimum dose application to healthy tissue should be always taken into consideration when employing radiation procedures. Moreover, epidemiological data show that inhabitants of countries with high background radiation such as India, China, the USA, and Japan are less likely to have cancer. It is possible that low-dose irradiations are associated with immune system activities; however, the precise health hazards of the exposure to low-dose irradiation are still unknown. Pramojanee et al., indicated that lower doses of radiation in dental radiography (e.g., peri-apical radiography) could have beneficial effects on osteoblastic cells by decreasing ROS formation, while higher doses of radiation in dental radiographies (e.g., 10 periapical radiographies) damaged the osteoblastic proliferation by a rise in ROS production (28). Arendash et al., revealed that prolonged exposure to electromagnetic fields could be used to treat some neurodegenerative disorders such as Alzheimer disease (27). Recent studies reported that short-term exposure to weak microwave radiation could temporarily activate specific immune responses, while long-term exposures may prevent specific immune responses (29). The main benefit of these physiological conditions is protecting cells and organs from harmful effects of future exposure to high-doses radiation. These physiological conditions are referred to as adaptive response. Mortazavi et al., stated that short-term exposure to extremely high levels of natural radiation (up to 196 times higher than the normal background) did not induce oxidative stress (30) and adaptive response (3). Feinendegen et al., suggested that adaptive response could be induced by ROS (31). Mortazavi et al., showed that short-term exposure to elevated levels of radon could stimulate an adaptive response in animals (32). Ciejka et al., stated that exposure to extremely low frequency magnetic field (ELF-MF) for 30 minute/day for 10 days can affect free radical generation in the brain and prolongation of its exposure (60 minute/day) elicited adaptation to this field. They also indicated that the time of animal exposure to magnetic fields affect oxidative stress parameters (33).
Therefore, the minimum level of damage induced by pre-exposure to ionizing and non-ionizing radiations by the increase of ROS levels could enhance resistance of living organisms (in vivo) or cells (in vitro) to higher levels of the same or other sources of stress; hence, ROS plays a key role in inducing adaptive responses. Moreover, ROS production rate, redox state along with previous exposure and other factors directly affect the induction of an adaptive response or oxidative stress. Other factors include: intensity of the frequency, exposure duration, metabolic activity, and tissue perfusion. Exposure to electromagnetic fields influence the releases of some hormones and neurotransmitters via changes in channel functions and transporters, concentration of Ca2+, and the structure of cytoskeletons (10).
3. Ionizing/Non-Ionizing Radiation, ROS, and the Damage to the Cellular Macromolecules
3.1. Source of ROS in Cell
The term reactive oxygen species refers to free radicals and their non-radical intermediates originated from oxygen (Table 1). Free radicals refer to species containing one or more unpaired electrons in the outer layer. Free radicals in biological systems can be generated from molecules of oxygen and nitrogen, which are in high concentrations in cells. Under physiological conditions, mitochondria are considered as the principal source and the superoxide anion is the most common oxygen free radicals in cells. In such conditions, about 2% of the oxygen consumed in cell is converted to superoxide anion by complexes of enzyme in respiratory chain. The transfer of electrons particularly complexes I and III is not efficient and results in the formation of superoxide anion. Under conditions of hyperoxia and raised glucose levels such as in diabetes, in which the activity of complexes of enzyme increases in electron transport chain, the formation rate of superoxide anion increases too. Also, about 25% of superoxide anion within the cells is generated by endoplasmic reticulum (ER). The production of superoxide anion within ER occurs by electron leakage from electron transport chain and the formation of disulfide bonds during protein folding (10, 34). Other sources of superoxide anion are under physiological conditions of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, cytochrome P450 oxidase, and other oxidoreductases (35).
| Radicals | Non-Radicals |
|---|---|
| Hydroxyl (OH°) | Peroxynitrite (ONOO°) |
| Superoxide (O2-) | Hypochloric acid (HOCL) |
| Nitric oxide (NO°) | Hydrogen peroxide (H2O2) |
| Thiyl (RS°) | Singlet oxygen(O-) |
| Peroxyl (RO2°) | Ozone (O3) |
| Lipid peroxyl (LOO°) | Lipid peroxide (LOOH) |
3.2. Radiation, ROS and Lipid Peroxidation
Polyunsaturated fatty acids (PUFAs) are considered as a type of fatty acids present in cellular phospholipids and organelle membranes with more than two carbon-carbon double bond(s); the adjacent of the double bond with methylene groups lead to the formation of ethylene carbon-hydrogen bond, which is weaker. Therefore, PUFAs are more susceptible to oxidation. The presence of PUFAs in plasma and organelle membranes is essential for membrane cross-sectional transport and fluidity, and maintains concentrations of nutrients and ions within the cell. PUFAs are the most susceptible macromolecules to oxidative damage by oxidants. Damage to PUFAs by ROS and other chemical substance cause impairment of membrane fluidity, ion pumps and carriers. The results lead to disruption of the physiological function of cells (10, 34).
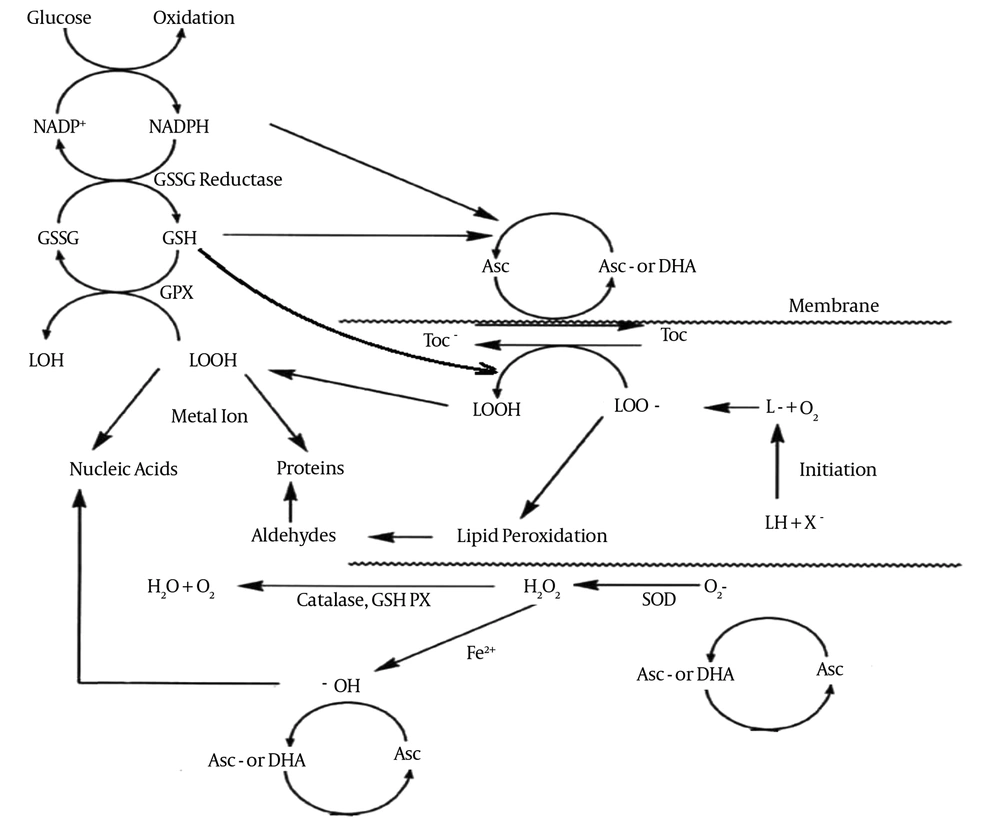
The damage to PUFAs by ROS is called lipid peroxidation three stages: initiation, propagation, and termination. Reaction of free radicals with PUFAs and other fatty acids release lipid-free radicals during initiation. Then lipid-free radicals react with molecular oxygen and release lipid peroxyl radicals, which react with PUFAs and fatty acids to generate lipid-free radicals; this process is called propagation. During termination, the two radicals interact, and the process comes to an end (35) (Figure 3). The process of lipid peroxidation especially initiation and propagation steps could affect the concentrations of binding proteins including ferritin, lactoferrin, albumin and ceruloplasmin, which determine the availability of transition metals such as iron and copper. The presence of metals has a critical role in the process (36).
Oxidative damages, especially lipid peroxidation, occur by ionizing radiations such as X and Gamma rays, alpha particles, beta particles, and neutrons. Exposure to gamma and X-rays initiates lipid peroxidation and increases malondialdehyde (MDA) in various tissue. MDA is a by-product of lipid peroxidation and is used in various biochemical assays to monitor the degree of per-oxidative damage sustained by cell. Radiotherapy during the course of cancer treatment damages normal tissue in approximately 50% of the patients. The rise in ROS in cells ensues within a period ranging from a few minutes to a few hours subsequent to radiation exposure (10).
3.3. Radiation, ROS and DNA Damage
Components of heredity substances of eukaryote cells are susceptible to oxidative damages; pyrimidines are more susceptible to oxidative damages than purines and DNA sugar (deoxyribose) (20). DNA damages by hydroxyl radicals via oxidation of DNA sugar are the main cause of base degradation, DNA fragmentation, and cross linking. Various studies showed that exposure to ionizing radiation directly and indirectly via generating singlet oxygen, induce lesions in cellular DNA through deletions, mutations and other lethal genetic effects (20). The 8-hydroxy-2-deoxyguanosine (8-OH-2-deoxyguianosine) is a common byproduct of DNA oxidation considered as a key biomarker of oxidative DNA damage (35).
Some studies show that pre-exposure of DNA and cells to lower doses of ionizing and non-ionizing radiations, oxidants, and heat cause primary damages, which protect the cells and heredity substances from detrimental effects of higher doses of these agents. Olivieri et al., reported that pre-exposure to lower doses of ionizing radiation in human monocytes decreased susceptibility to chromatid break induced by a subsequent high-dose radiation (37).
The physiological roles of mitochondria, such as energy metabolism or free-radical generation are closely associated with the normal activity of cells; hence, direct damage to mitochondria by exposure to radiation or indirect damage via oxidative damages can be one of the causes of cell dysfunction (10). Oxidative damage to mitochondrial DNA directly or indirectly, after exposure to ionizing and non-ionizing radiations, cause neurotoxicity closely associated with various nervous system problems such as Alzheimer disease.
High intra-mitochondrial ROS level, either via damage to mitochondrial DNA or by decreasing the activity of methyltransferases, affects the epigenetic control of the nuclear DNA (16) (Figure 3). Trosic et al., revealed that exposure of brain, liver, and kidney of the rat to radiofrequency electromagnetic radiation (915 MHz, power density of 2.4 W/m2 and SAR of 0.6 W/kg) could cause DNA breaks (38). Xu et al., indicated that exposure of cultured neurons to radiofrequency radiation (1800 MHz) could cause oxidative damage to mitochondrial DNA (39). Therefore, the function and structure of mitochondria can be considered in the treatment of many diseases.
3.4. Radiation, ROS, and Damage to Protein
Ionizing and non-ionizing radiations generate oxygen free radicals such as singlet oxygen, which can react with five amino acids tryptophan, histidine, tyrosine, methionine, and cysteine. The reaction rate of singlet oxygen with these amino acids depends on the number of double bonds of thiol groups. Tryptophan, histidine, and tyrosine contain double bonds and directly react to singlet oxygen. Methionine and cysteine contain a sulfur atom with four non-pair electrons that react to singlet oxygen. The reaction rate of ROS and proteins depend on pH, temperature, and dielectric of the cellular environment (36). Oxidative damage to proteins is affected in the presence of metal cofactors that are capable of redox cycling such as Fe, Cu, and Zn (36).
4. Antioxidants and Their Strengthening Strategies
The antioxidant defense systems controlling the balance between the production and elimination of free radicals in cells are specific and efficient. The antioxidant defense systems include enzymatic and non-enzymatic antioxidants. The detoxification process by antioxidants involves electron transfer from antioxidant molecules to free radicals or vice versa. Enzymatic antioxidants are one of the most important intracellular defense mechanisms. The antioxidant enzymes have one or more transition metals in their cores that transfer electrons during detoxification process. The enzymes of superoxide dismutase (SOD) group are the first antioxidant defense system against free radicals. Copper-zinc SOD (Cu-Zn SOD) is located in cytosol and manganese-SOD (Mn-SOD) is located in mitochondria. Extracellular-SOD is another form of SOD located outside the cells (35). They convert superoxide anions to H2O2 and molecular oxygen. In the next stage, the catalase converts H2O2 to water. Glutathione peroxidase reduces hydroperoxides; especially lipid hydroperoxides during lipid peroxidation, which depends on the presence of recovered glutathione (GSH). GSH is synthesized from L-cysteine, L-glutamate, and glycine in cytosol and acts as a hydrogen donor. It is one of the major endogenous antioxidants and acts as a thiol redox buffer in cells. Moreover, physiological concentration of GSH is necessary to maintain structural integrity and physiological functions of cell membranes. The conversion of GSH to glutathione peroxidase is done by glutathione reductase in the presence of NADPH. Pentose phosphate pathway by glucose-6-phosphate dehydrogenase generate NADPH; hence, any damages to this enzyme can lead to oxidative damage. ROS, electrophilic chemicals, and radiation can cause cellular and DNA damages which can be protected by GSH. Exposure to 50 Hz ELF-MF can change redox state by altering the levels of GSH (40). Ascorbic acid (vitamin C) and α-tocopherol (vitamin E) are the main components of non-enzymatic defenses. These vitamins are essential nutrients for humans. These vitamins as a part of non-enzymatic antioxidant defenses have various functions and act as natural antioxidant and pro-oxidant. Vitamin C is an antioxidant that quenches hydrogen peroxide, and ameliorates the activity of catalase and glutathione peroxidase. The functions of vitamin C as an antioxidant or pro-oxidant are affected by the local concentrations of this vitamin, the redox potential of the cellular environment, and the presence/absence of transition metals (41). Studies show that vitamin C has antioxidant activity in a dose-dependent manner. Kumar et al., showed that vitamin C (100 mg/kg body weight/day) has an antioxidant role (42). It was also shown that vitamin C (200 mg/kg body weight/day) improves antioxidant enzymes activity and decreases lipid peroxidation in various tissues and prevents oxidative stress (41).
Vitamin C at pH 7.0 has a very low standard reduction potential (282 mV); therefore, GSH, α-tocopherol, NADH, and NADPH can regenerate vitamin C (36). The presence of transition metals for example iron and copper is considered important in oxidative damages such as Fenton reaction and the initiation and propagation steps of lipid peroxidation (41). Cai et al., also indicated that vitamin C, in the presence of copper, has high antioxidant activity against radiation-induced DNA damages (43).
Vitamin E or α-tocopherol is one of the most important antioxidants in human body. Natural antioxidant can protect membrane lipids, particularly PUFA, from oxidative damage induced by chemical substances (44) and radiations (45); hence, the major antioxidant function of vitamin E is inhibiting lipid peroxidation. Antioxidant activity of α-tocopherol was done by the transfer of a hydrogen atom at 6-hydroxyl group and scavenging of singlet oxygen and other ROS (36). The use of alpha-tocopherol supplementation in human and animal studies decreases lipid peroxidation and superoxide production by imbibing the activity of NADPH oxidase as well as decreasing the expression of scavenger receptors (SR-A and CD36), particularly in foam cells. It was also shown that α-tocopherol therapy, especially at higher doses, could decrease the release of pro-inflammatory cytokines, interleukin-8 and the plasminogen activator inhibitor-1 levels as well as adhesion of monocytes to endothelium (46).
5. Conclusions
Exposure to ionizing (e.g., solar radiations, cosmic and X-rays) and non-ionizing radiations (e.g., RFW emitted from mobile phones and wireless) impair cellular structure and function through changes in the structure and functions of the cell membrane, extracellular/intracellular signaling pathways, genetic effects, oxidative stress, and DNA damages. The imbalance between the production of ROS and antioxidant defense mechanisms is the oxidative stress. Ionizing and non-ionizing radiations, environmental stimuli such as environmental toxins, altered atmospheric conditions (e.g., hypoxia and hyperoxia), inflammation and infections enhance ROS production. ROS through interference in cell signaling pathways, DNA and proteins damages, and lipid peroxidation can cause oxidative damages or protect the cells against these damages by adaptive response. Oxidative stress causes cellular dysfunctions. Based on the results of several studies, the production rate of ROS, redox state of cells, previous exposure to harmful agents along with other factors affect the induction of adaptive responses or oxidative stress. By finding proper mechanisms for oxidative stress and by professional diagnostic techniques, appropriate solutions can be found to decrease cellular dysfunctions and diagnose many disorders.