1. Background
Today, living in advanced societies is closely linked to various lifestyle stressors, such as exposure to UV radiation and radiofrequency waves, peripheral pollutants, cigarette smoking, and excessive alcohol consumption (1). These lifestyle stressors influence human health through various ways, in particular oxidative stress. Over-consumption of alcohol, after smoking and hypertension, is one of the most common risk factors for disease burden in high-income countries (2). However, consumption of alcohol has been popular in many societies and it seems to continue to be so in the future. Excessive alcohol consumption can have important health outcomes. Alcohol consumption is associated with the development of chronic diseases, such as liver disease, diabetes mellitus, heart disease, cancer and bone diseases, and increases their occurrence (3, 4). Liver has various functions, such as metabolism, storage, secretion, generation of plasma proteins, biochemical processes of growing, providing nutrients, supplying energy, reproducing, and detoxification of waste metabolites and toxic compounds (5). The liver as a central core plays a critical role in the metabolism of ethanol in the process of the first passage via a simple oxidative pathway, especially alcohol dehydrogenase (ADHs) pathway in the cytosol of the cell. The microsomal ethanol-oxidizing system is another oxidation pathway of ethanol, which is allocated in the smooth endoplasmic reticulum; in this pathway, ethanol was metabolized by cytochrome P450 (Cyt P450) and utilizes NADPH and molecular oxygen, and these pathways generate acetaldehyde and reactive oxygen species (ROS) (6). Over-consumption of ethanol via oxidative stress and ROS-mediated toxicity can cause structural and functional changes in liver cells and play a central role in the development of alcoholic related diseases, especially alcoholic liver disease (ALD). Furthermore, ALD has a frequent association between chronic alcoholism and diverse hepatic lesions. Hepatotoxic chemicals by oxidative damages lead to a considerable amount of liver damage (7). The use of medicinal plants and herbal products today has gained popularity in preventing and treating many diseases, including cardiovascular (8), respiratory (9), reproductive (10), and hepatic (11) diseases, via producing secondary metabolites such as proteins, steroids, flavonoids, phenols, and other bioactive compounds (7). Also, in some cases, high efficacy, low cast, and low incidence of side effects are other advantages of herbal medications. Ginger (Zingiber officinale) is a well-known medicinal herb, which contains numerous phytochemical compounds, such as polyphenols including flavonoids and tannins with anti-inflammatory (12), antioxidant (10), anti-carcinogenic (13), anti-diabetic, and hypo-lipidemic (14) activities. Ginger has antioxidant properties due to zingerone, gingerdiol, zingiberene, gingerols and shogaols (13). It has been prescribed in traditional Persian medicine as a hot-inducing remedy. It was described in “The Canon of Medicine” by Avicenna (980 - 1037 AD) and “The Storehouse of Medicaments” of Aghili Shirazi (written in 1772 AD) as a herbal remedy for several diseases, including headache, hepatic disorders, and gastrointestinal diseases (15).
2. Objectives
The aim of the current study was to evaluate the hepatoprotective effects of ginger extract against ethanol-induced deterioration in biochemical parameters, including aspartate amino-transferase (AST), alanine amino-transferase (ALT), total triglycerides (TG) and total cholesterol (TC) for evaluating liver functions. Furthermore, hepatic antioxidant enzymes, including catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD) and malondialdehyde (MDA) as a marker of oxidative stress, were assessed to evaluate the protective effect of ginger against ethanol-induced oxidative damages in liver functioning of male rats.
3. Methods
3.1. Preparation of Ginger (Zingiber officinale Roscoe) Extract
Rhizome of fresh ginger was purchased from a herbal shop (Attari) in Shiraz, Iran. It was verified by a botanist in Shiraz University of Medical Sciences and recorded in the university herbarium (Voucher number: PM-948). The process of preparation of ginger extract was done, as previously described (16).
3.2. Phytochemical Analysis
3.2.1. Evaluation of Total Phenolics Content (TPC) and Total Antioxidant Activity
Evaluation of extract total phenol content was done using the modified Folin-Ciocalteu spectrophotometric method, as described by Waterhouse (2002) (16). Briefly, 40 μL of the prepared extract, 200 μL Folin-Ciocalteure agents, and 3.16 mL of water were mixed for eight minutes. The amount of 600 μL of sodium carbonate (0.25%) was added to this solution. Then, it was incubated for two hours at room temperature in darkness. The measured absorbance by a UV-visible spectrophotometer was 765 nm in comparison to the blank. Standard solution of gallic acid also underwent this procedure and the calibration standard curve was interpreted, accordingly. Comparison was done between the measurement and a gallic acid solution standard curve. Total phenolics concentration was remarked as milligrams of gallic acid in each gram of dry extract (mg of GA/g of dE).
Evaluation of extract total antioxidant capacity was done using a modified method, as described by Leong and Shui (17). Briefly, diphenyl picryl hydrazide (DPPH) is a compound, which comprises stable free radicals. It could accept an electron or radical hydrogen and become yellow-colored. Antioxidant-induced decrease in the absorbance at 517 nm has been used for estimation of the reduction capacity of DPPH. Measurement of DPPH scavenging activity was done spectrophotometrically. A fresh methanol solution of DPPH (0.3 mM) was prepared. An amount of 0.1 mL of the samples and 3.0 mL of the prepared solution were mixed. Test tubes were shaken well in addition to their 30-minute incubation at room temperature and dark. Absorbance measurement at 517 nm was conducted considering methanol as the blank. In addition, tubes containing 3.0 mL of DPPH reagent and 1.0 mL of methanol were considered as control for absorbance.
3.3. Animals
All investigations were conducted in accordance with the "Guiding Principles for the Care and Use of Research Animals" approved by Shiraz university and European Council Directive (86/609/EC) of November 24th, 1986.
Twenty-four adult male Sprague-Dawley rats (220 ± 15 g) were colony-bred (six rats per cage) in the animal room under controlled lighting (12 hours light: 12 hours darkness) and temperature (20°C ± 2°C) conditions and had free access to a pelleted food and tap water.
3.4. Experimental Design
To evaluate the effects of ginger extract on function of liver and indices of oxidative stress against toxicity induced by ethanol in this tissue, animals were randomly allocated to four equal groups (n = 6 in each group).
Group I: Vehicle or control group, which received normal saline (1 mL/day)
Group II: Ethanol group, which received ethanol (4 g/kg of body weight (B.W)/day)
Group III: Ginger group was assigned to receive ginger rhizome extract (1 g/kg of B.W/day)
Group IV: Ginger-ethanol group, which received ethanol (4 g/kg of B.W/day) after administration of ginger (1 g/kg of B.W/day).
Daily oral administration of the vehicle and extract were done between 09:00 AM and 09:15 AM for 28 days. Razi Chemical Company (Tehran, Iran) was chosen for purchasing alcohol (ethanol 96%).
3.5. Biochemical Analysis
At the end of the study, the rats were fasted overnight with free access to drinking water. They were sacrificed anesthetizing with ether (Merck Chemical Company, German) and further by cervical dislocation. Blood samples were collected via heart puncture and whole blood was placed in sterile micro tubes and then centrifuged at 3000 g for five minutes and serum was collected in a new micro tube and stored at -70°C for assaying liver enzymes activity. Immediately after collection of blood, liver was removed and rinsed in ice saline. The tissue was rapidly homogenized manually in cold phosphate buffer (pH = 7.4, 0.1 M) and debris removed by centrifugation at 3000 g for 10 minutes. The upper clear supernatants were then recovered and stored at -70°C for assaying antioxidant enzyme activity and total protein.
3.6. Biochemical Parameters Measurement
The levels of liver enzymes (ALT and AST), total cholesterol (TC) and total triglycerides (TG) as biochemical parameters for evaluating liver function were assayed, according to the manufacturer’s instructions using kits supplied by Pars Azmun Co. (Tehran, Iran).
3.7. Assessment of Liver Oxidative Status
To evaluate the ethanol-induced oxidative damage in liver tissue, antioxidant enzymes activity and malondialdehyde were measured. Antioxidant enzymes included total superoxide dismutase, glutathione peroxidase and catalase. The activity of total superoxide dismutase and glutathione peroxidase were determined according to the manufacturer’s instructions, using laboratory Ransel and Ransod kits (Randox Company, UK), respectively. To evaluate catalase activity of the liver, the researchers used a method described by Aebi (18). Briefly, 0.5 mL of 30 mmol/L H2O2 solution in 50 mmol/L phosphate buffer (pH = 7.0) and 1 mL of 1:10 diluted tissue supernatant was added and the consumption of H2O2 was followed spectrophotometrically at 240 nm for two minutes at 25°C. The molar extinction coefficient was 43.6 l/mol per cm for H2O2. Catalase activity was expressed as the unit that is defined as μmol H2O2 consumed/min per mg tissue protein.
The MDA level were evaluated by a modified HPLC method, which is based on the reaction of MDA with thiobarbituric acid (TBA) to form a colored MDA-TBA adduct (19). Briefly, 0.5 mL of tissue supernatant was added to 2 mL TBA reagent containing 0.375% TBA, 15% trichloroacetic acid, and 0.25 mol/L HCl. The mixture was immediately heated (60 minutes at 95°C) and cooled with running water and thereafter butanol-pyridine (15:1, v/v) (1 mL) was added and the final volume was adjusted to 2 mL with distilled water. After vigorous mixing, the organic layer was separated by centrifugation (16000 g, three minutes, at room temperature). The supernatant was analyzed on a UV-visible spectrophotometer fitted with an 80-μL flow cell (20, 21). The absorbance was measured at 532 nm (the mobile phase consisted of 300 mL/L methanol in 50 mM KH2PO4, pH = 7.0). Furthermore, 1, 1, 3, 3-tetraethoxypropane was used as a standard, and MDA-TBA reactive substance values were expressed as nmol per milligram of tissue protein (nmol/mg protein). The HPLC system consisted of a solvent delivery pump (Jasco 980-PU, Tokyo, Japan), a reversed-phase column (Luna C18, 250 mm × 4.6 mm, Phenomenex, CA, USA), and a UV-Vis detector (Jasco, UV-975, Tokyo, Japan) operated at 532 nm.
3.8. Protein Content
The total protein concentration of tissue homogenates was determined according to Lowry et al. (20).
3.9. Statistical Analysis
The descriptive results were expressed as means ± standard error of mean (Mean ± SE). All data were recorded with the statistical package for social sciences (SPSS17.0). The results were analyzed using one-way analysis of variance (ANOVA) followed by post Hoc multiple comparisons Tukey test for comparison between different treatment groups. Repeated measures analysis was used in weight comparison. Statistical significance was set at P < 0.05.
4. Results
In this study, the Phytochemical analyzes of hydroalcoholic extract of Zingiber officinale (ginger) showed large amounts of phenolic and flavonoid compounds and high antioxidant capacity. The DPPH inhibition of the sample was expressed as the percentage of inhibition. Hydroalcoholic extract of ginger possessed the reasonable DPPH scavenging activity (IC50 ¼ 75.0% + 1.41% mg/mL inhibition of the DPPH radical).
The mean values (+ SE) of body weight, liver weight, and liver index are presented in Tables 1 and 2. Administration of ethanol significantly decreased body weight, while increased liver weight and liver index in the ethanol group compared to the control group. Administration of ginger corrected body weight, liver weight, and liver index to the normal level in the ginger-ethanol group (P < 0.05) with no significant difference compared to the control group (P > 0.05) (Tables 1 and 2).
| Group | Control | Control + Ginger | Ethanol | Ginger + Ethanol |
|---|---|---|---|---|
| Day 0 | 260.57 ± 7.14aA | 265.77 ± 4.60aA | 262.17 ± 3.16aA | 274.17 ± 4.36aA |
| Day 7 | 278.73 ± 7.28aA | 273.48 ± 4.67aA | 248.34 ± 3.37aA | 254.33 ± 4.42aA |
| Day 14 | 286.47 ± 7.59abA | 283.23 ± 4.58abA | 232.73 ± 3.16abB | 264.33 ± 4.2aA |
| Day 21 | 296.80 ± 7.79bcA | 299.91 ± 6.80bcA | 216.87 ± 3.11bcB | 271.89 ± 4.21aA |
| Day 28 | 321.17 ± 7.61cA | 326.27 ± 6.74cA | 199.79 ± 2.99cdB | 279.25 ± 3.90aC |
aDifferent capital alphabetic letters show significant differences between groups. Different small alphabetic letters show significant differences between days of evaluation (P < 0.05).
| Group | Control | Control + Ginger | Ethanol | Ginger + Ethanol |
|---|---|---|---|---|
| Liver weight, g | 11.89 ± 0.47A | 12.13 ± 0.34A | 8.78 ± 0.52B | 10.93 ± 0.45A |
| Body weight, g | 321.17 ± 7.61A | 326.27 ± 6.74A | 199.79 ± 2.99B | 279.25 ± 3.90C |
| Liver index (LW/BW), % | 3.7 ± 0.06A | 3.71 ± 0.05A | 4.39 ± 0.17B | 3.91 ± 0.11A |
aDifferent capital alphabetic letters in row show significant difference between groups (P < 0.05).
The mean values ( ± SE) of serum ALT, ASL, and lipid levels as biomarkers for liver function in the experimental groups are presented in Table 3. The results in the ethanol group showed that oral administration of ethanol significantly increased biomarkers (P < 0.05, Table 3), while ginger extract in the ginger + ethanol group decreased levels of the liver enzymes and lipids and prevented detrimental effects of ethanol on them (P < 0.05, Table 3). The levels of serum LDL, TG, and TC as lipid profiles in the ethanol group increased significantly compared to other groups (P < 0.05), while levels of these factors in ginger-ethanol group were significantly decreased in pretreatment with ginger compared to the ethanol group (P < 0.05, Table 3).
| Group | Control | Control + Ginger | Ethanol | Ginger + Ethanol |
|---|---|---|---|---|
| ALT, IU/L | 60.25 ± 3.45A | 59.68 ± 5.60A | 154.28 ± 7.84B | 100.87 ± 3.34C |
| AST, IU/L | 178.73 ± 12.28A | 166.48 ± 8.64A | 248.78 ± 6.44B | 204.26 ± 4.42C |
| TC, mg/dL | 28.58 ± 7.59A | 28.35 ± 4.58A | 68.86 ± 3.16B | 45.87 ± 6.1C |
| TG, mg/dL | 65.45 ± 5.19A | 62.51 ± 3.80A | 98.17 ± 7.11B | 70.24 ± 3.51C |
| LDL, mg/dL | 62.57 ± 7.61A | 60.27 ± 6.74A | 90.45 ± 2.99B | 71.25 ± 3.90C |
aValues are expressed as mean ± SE.
bDifferent capital alphabetic letters in row show significant difference among groups (P < 0.05).
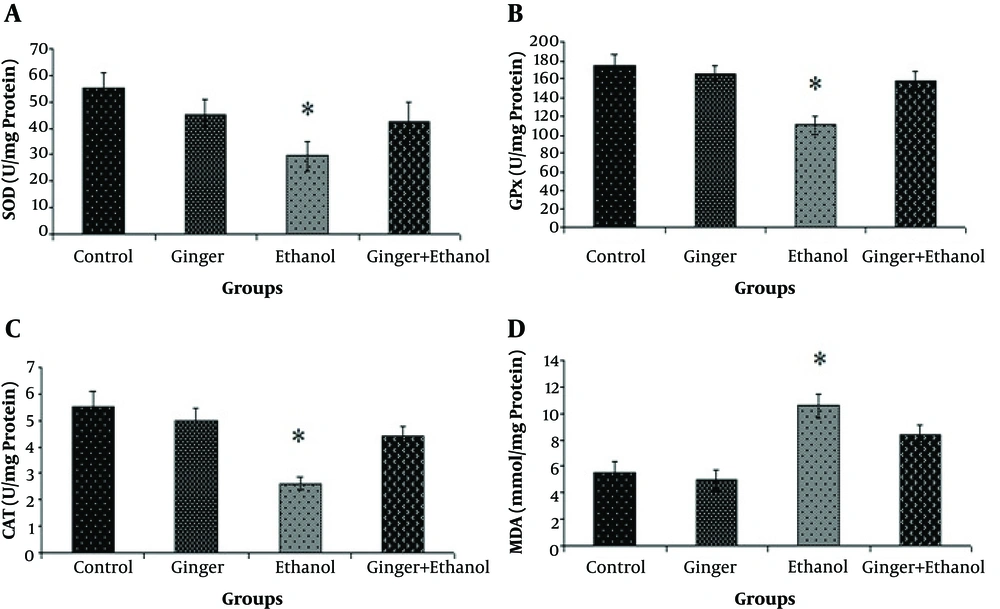
The protective effects of ginger extract on liver oxidative status in hepatotoxicity induced by ethanol are shown in Figure 1. The mean (± SE) activity of antioxidant enzymes (SOD, GPx, and CAT) was significantly elevated after administration of ethanol in the ethanol group compared to other groups (P < 0.05, Figure 1), while pretreatment with ginger returned their levels to normal (P < 0.05, Figure 1A - C). The level of MDA as one of the byproducts of lipid peroxidation was measured for the evaluation of oxidative stress. In the ethanol group, MDA level increased significantly compared to other groups, while pretreatment with ginger significantly decreased it compared to the control group (P < 0.05, Figure 1D).
Comparison of antioxidant enzymes activity (A, total superoxide dismutase (SOD); B, glutathione peroxidase (GPx); C, catalase (CAT); and D, malondialdehyde (MDA)) among the control and treated rats (n = 6). Data are expressed as mean ± SE of enzyme activity (U/mg protein of liver tissue). Asterisk shows a significant difference among groups (P < 0.05).
5. Discussion
The results of phytochemical analysis showed that the hydroalcoholic extract of ginger had high level of phenolic compounds, which was able to produce its high antioxidant capacity. The antioxidant activity of the hydro-alcoholic extract of ginger refers to the presence of phenolic, flavonoids, carotenoids, and tannins in this plant, which can serve as a natural source for free radical scavengers (21). The results of this study showed that ethanol consumption could significantly reduce body weight and liver weights in the ethanol group, whereas pretreatment with ginger extract can improve them and somewhat brought them to normal levels. The decrease in liver weight can be due to damage to liver cells and liver cells organelles, especially mitochondria by over-consumption ethanol (3). Liver damage caused by over-consumption ethanol can be due to the development of liver diseases, such as fatty liver, ADL, alcoholic hepatitis, and cirrhosis. Chavez et al. indicated that ethanol in high physiological doses impairs normal hepatocyte proliferation via reducing (insulin-like growth factor-1) IGF-1 levels (22); ethanol in physiological concentrations inhibits IGF-1-mediated signaling and proliferation of C6 rat glioblastoma cells (23). Ethanol also alters energy metabolism in the liver (24) and can cause body weight loss. Increased liver index in the ethanol group can be due to the accumulation of fat and degeneration in the liver. This result was in agreement with a previous report (25). However, the results of the ginger-ethanol group showed that pretreatment with ginger extract could increase body weight and liver weight. Studies showed that ginger can influence body weight through the control of thermogenesis, food intake, and levels of ghrelin and leptin (26, 27). Also, in spite of the presence of high level of flavonoids, phenols, and others, the protective effects of ginger may be due to proteins and small peptides, which can improve body weight; it is suggested that these compounds activate the central serotonin signaling pathways, which control satiety (28), so that it can be a therapeutic target to control obesity. Regeneration and recovery of liver structure and function can play a critical role in improving body weight and the current results showed that ginger could significantly accelerate the recovery of the liver damage by ethanol and increased body weight. The results of this study showed that administration of ethanol in the ethanol group could increase MDA and decrease antioxidant enzymes activity in the liver compared to the control group, while pretreatment with ginger extract could increase the activity of antioxidant enzymes and reduce lipid peroxidation in ginger-ethanol group, which is in agreement with previous reports (10). Reduction in liver GSH, antioxidant enzymes, and immunoreactive protein levels and increase in superoxide and hydroxyl radicals by cellular metabolism of ethanol leads to oxidative damage and dysfunctions (4). Ethanol either changes in the post-transcriptional synthesis of antioxidant enzymes or stimulates their intracellular degradation and leads to oxidative stress (4). It is well known that the main damage to cells occurred by high levels of ROS, which reacts to lipids, proteins and DNA, and can induce apoptosis, which causes tissue injury (3). Overproduction of NADPH and ROS after ethanol consumption could cause damage to liver cells organelles, such as mitochondrial and change in energy metabolism (29). Mitochondrial oxidative stress induced by ethanol can cause overproduction of superoxide anion (O2•−) at respiratory chain complexes I and III, and consequently the production of other ROS, which was triggered by NAD(P)H overproduction (30). Consequently, ROS may react with lipids of membranes in the cell and cellular organs and increase lipid peroxidation, which influences the permeability of membrane. Recent studies have also shown that long-term consumption of ethanol could increase the permeability of the membrane, reducing the mitochondrial membrane potential, and impairing the activity of mitochondrial respiratory chain complexes, as a result impair ATP synthesis (4). Accordingly, these pathways can be considered as potential therapeutic targets for alcohol abusers.
The current study found that pretreatment with ginger could improve oxidative status in liver tissue so that it increased the level of its enzymes. Reactive oxygen species and nitric oxide are responsible for the induction of hepatocyte apoptosis (3). The elevation in AST and ALT reflects hepatocellular injury (11). The results showed that ethanol can increase the levels of these enzymes in serum, while pretreatment with ginger can improve the levels of hepatotoxicity induced by ethanol. It was known that metabolism of ethanol in the liver was associated with changes in structure and function of this organ, which causes release of these enzymes to blood (11). However, biomarkers for evaluating oxidative stress (SOD, GPx, CAT, and MDA) showed that pretreatment with ginger extract can improve liver structure and function. Many studies showed that pretreatment with ginger significantly improved liver structure and reduced MDA activity in liver damage induced by thioacetamide (31) and adriamycin (32).The antioxidant properties of ginger comes from zingerone, gingerdiol, zingiberene, gingerols, and shogaols (21), which act as scavengers of free radical and inhibit lipid peroxidation and damage to DNA, which could improve hepatocyte structure and function, such as proliferation (11, 32). However, ginger may have a high level of flavonoids, phenols, zingerone, gingerdiol and others, and the hepatoprotective effects may be presence of proteins and small peptide. In the present study, the results showed that administration of ethanol can increase the level of TG, TC, and LDL in serum as biomarkers for liver function, while pretreatment with ginger can improve their level. Studies showed that long-term ethanol consumption causes changes in the structure and function of liver, especially synthesis of lipoproteins and plasma proteins of liver so that level of total protein in the study increases significantly in the ethanol group. It was reported that lipoproteins and plasma proteins, such as albumin and Apo lipoproteins play a role in lipids metabolism, and increase levels of TG, TC, and LDL in this study and can be due to dysfunction of hepatic lipids metabolism by excessive ethanol consumption. The results of this study showed that ginger can decrease their level. Animal models for diabetes and atherosclerosis indicated that daily oral ginger decreased the serum levels of FBS, LDL, TC, and TG, and increased serum HDL-C level (33). Ginger via reducing plasma cholesterol and by inhibiting LDL oxidation inhibited atherosclerosis in animal model (34). The hypocholesterolemic effects of ginger may be due to presence of (E)-8 beta, 17-epoxyllabed-12-ene-15, 16 dial compound, which inhibits cellular cholesterol synthesis (35), and reducing LDL oxidation by ginger occurs via scavenging superoxide anion and hydroxyl radicals (36).
5.1. Conclusions
It should be noted that liver is an important organ because it plays a critical role in first passage for ethanol and ginger. The findings of this study indicated that pretreatment with ginger can improve body weight, liver weight and liver index, oxidative stress, and liver function in ethanol-induced hepatotoxicity in male rats. This finding is consistent with other traditional use of ginger for controlling antioxidant enzymes activity and liver function.