1. Background
Mesenchymal Stem Cells (MSCs) are multipotent, non-hematopoietic stem cells that can make several types of mesenchymal lineages (1). These calls also have valuable immunoregulatory properties, which can provide a valuable approach to control graft-versus-host diseases and autoimmune disease (2). Mesenchymal stem cells have been recognized to possess immunoregulatory properties, as they straight network with a lot of lymphocytic, leukocytes, and myeloid cells; consequently, these cells may be considered for memorable methods of cell-based immunotherapy (3).
Of note, MSCs function can be affected by a wide range of environmental factors (4-6). They represent all four adenosine receptor subtypes. Therefore, adenosine possesses an important role in MSC proliferation and differentiation (7). Moreover, phosphodiesterase inhibitors like tadalafil can protect MSCs against hypoxia/reoxygenation damage via STAT3/PKG-I signaling (8). Currently, MSCs have particularly been used in cell remedy tasks for inflammatory and autoimmune conditions, therefore, they have immune changes properties along with re-generative potency (9). On the other hand, MSCs implanted in the peri-vascular and peri-endothelial areas can directly communicate with neutrophils in the inflammatory condition (10, 11). Mesenchymal stem cells in the bone marrow and other tissues have unpreventable associations with hematopoietic cells like macrophages and neutrophils.
Neutrophils are the most abundant invader-fighting cells in living multicellular organisms and appear early in the evolution from slime molds to primates (12). Neutrophils contain cytotoxic granules and can eliminate invaders via cytotoxic granules, reactive radical species production (13), and neutrophil extracellular trap (NET) formation (14).
It is known that 3,7-dimethylxanthine or theobromine is a bitter, herbal purine alkaloid derived from xanthosine, which can be found in the cacao plant, tea, kola nut, chocolate, and certain medicines (15). Xanthine alkaloid has multiple effects on different body systems, like cardiovascular, endocrine, respiratory, gastrointestinal, urinary, metabolism, and especially immune and central nervous systems (16, 17). The structure of methylated xanthine derivatives like theobromine is similar to that of adenosine. More importantly, theobromine is one of the famous competitive inhibitors of adenosine (18, 19). Theobromine also is a non-selective, competitive inhibitor of phosphodiesterase and hence, can increase the level of intracellular cyclic adenosine monophosphates (20).
The crosstalk between MSCs and neutrophils has been reported in some valuable studies (21-24). Nonetheless, there are no or very little data about the role of theobromine as a routine food material in the crosstalk between MSCs and neutrophils. With rising evidence of the role of MSCs in the immediate modulation of the innate arm of the immune system, MSCs treatment is gradually being regarded as a modern and interesting therapy for diseases like severe lung damage (25), bacterial infection (26), and sepsis (27).
2. Objectives
Here, we examined the inflammatory functions of neutrophils after co-culture with the conditioned MSC Medium (CM) whose MSCs had previously been pulsed with theobromine.
3. Method
3.1. Materials
Fetal calf serum (FCS), RPMI-1640, and Dulbecco’s Modified Eagle’s Medium (DMEM) were obtained from GIBCO/Life Technologies Inc. (Gaithersburg, MD). Dextran was bought from Fresenius Kabi (Verona, Italy). Theobromine and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. Isolation and Expansion of Mesenchymal Stem Cells
Mesenchymal stem cells were isolated as described elsewhere (28). In brief, the aspirated bone marrow from the femurs and tibias of deeply anesthetized Wistar rats was washed and plated in tissue culture flasks with concentrations of 0.3 to 0.4 × 106 cells/cm2 in DMEM, supplemented with 10% FCS. Non-adherent isolates were removed four days after initiation and the remnant adherent cells were fed every other day. After the cultures reached 75% - 80% confluence, trypsin/EDTA was used for the passage of cells. The immunophenotyping of the third subculture of MSCs was performed with antibodies against rat antigens including CD45, CD90 (Thy-1/Thy-1.1-FITC), CD29 (Integrin b chain; Ha2/5; FITC), and their isotype controls (IgG2a; FITC) as described previously (2).
3.3. Isolation T Cell and T Cell Proliferation Assay
Mononuclear cells (MNCs) from PBMCs were isolated using Ficoll-Hypaque density gradient centrifugation (Biochrom, Berlin, Germany). A single-cell suspension from MNCs was plated to eliminate the adherent cells. Cells in suspension were re-suspended in RPMI-1640 medium Nylon wool column, wetted with RPMI-1640 medium, and packed with the single-cell suspension. Then, T lymphocytes were gathered as elute and rinse from the nylon wool column based on no adherence. Consequently, for T cell proliferation assay, 100 × 105 T cells were seeded into 96-well plates and a group as control added PHA and for test group T cell (100 × 105 cells) co-culture with (10 × 105 cells) of MSCs. Cell numbers were defined by cell count reagent SF (Nacalai Tesque) following the manufacturer’s guidelines by measuring absorbance at 450 nm (SpectraMax M5 Microplate Reader).
3.4. Priming of MSCs with Theobromine and Isolation of Supernatants
The third subculture of MSCs was incubated with various concentrations of theobromine (0, 10, 50, and 100 µM) for 48 hours. After aspiration of the medium, MSCs were washed three times with PBS and incubated in a serum-free culture medium for 24 hours. The isolated supernatant was centrifuged at 300 g for 10 minutes and filtered via a 0.2-μm filter to remove the cellular debris.
3.5. Neutrophil Isolation and Co-culture with CM Isolated From MSCs
Cardiac puncture under deep anesthesia was used to isolate citrated blood samples. After centrifugation, the buffy coat was exposed to dextran sedimentation (1% w/v), followed by centrifugation on a Ficoll-Hypaque density gradient. The mononuclear cells and the plasma were omitted. Red blood cells were destroyed by hypotonic lysis. The isolated neutrophils were rinsed and suspended in the culture medium (4). Following this procedure, the purity of neutrophils was 95%. Afterward, 2 × 106 neutrophils were cultured in the medium containing 50% CM at 37°C for 4 hours. Finally, neutrophils were gathered, washed, and used for the next examinations.
3.6. Assessment of Neutrophils Viability
The neutrophils’ viability was evaluated using the MTT assay, similar to the methods described earlier (29, 30). The experiments were performed in triplicate.
3.7. Neutral Red Uptake
The natural red uptake assay offers a quantitative estimation of the number of viable cells in culture. It is predicated on the power of viable cells to include and bind the supra-vital dye natural red in lysosomes. Briefly, 10 μL of the NR solution (0.33%) was added to 100 µL of the neutrophil suspension (2 × 106 cell/mL) for 2 hours at 37°C. The medium was discarded and neutrophils were twice rinsed. Then, 100 µL of acetic acid (10%) plus 40% ethanol solution was used to solubilize the internalized NR. The optical density was monitored at 550 nm (31).
3.8. Phagocytosis Assay
This procedure was done as previously mentioned, with some modifications (32). In short, neutrophils were rinsed after stationary incubation of cells with opsonized yeast for 1 hour at 37°C, cytocentrifuged onto glass slides, and finally fixed in methanol. May-Grunwald-Giemsa was used to stain the slides and the yeast uptake was evaluated using light microscopy under oil immersion. The phagocytic ability of neutrophils was reported as the number of neutrophils that engulfed at least one yeast.
3.9. Respiratory Burst
To check the generation of reactive oxygen species (ROS) by neutrophils, the NBT reduction assay was performed (33). In brief, neutrophils were cultured alone or with CM for 4 hours. Then, neutrophils were kept for 20 minutes with 100 ng/mL phorbol 12-myristate 13-acetate and 0.1% NBT. The un-used dye was omitted by three times washing. Reduced NBT was extracted in dioxin and spectrophotometrically monitored at 520 nm.
3.10. Myeloperoxidase Assay in Neutrophil Supernatants
Neutrophils were cultured alone or with CM for 4 hours and then, they were pulsed with 100 ng/mL phorbol 12-myristate 13-acetate for 20 minutes. The supernatant of neutrophil culture was collected and used for the Myeloperoxidase (MPO) assay based on the protocol described earlier (34). Of note, in each experiment, the level of MPO activity in CM before co-culture with neutrophils was determined and subtracted from the finally calculated values.
3.11. IL- Condition Medium of MSCs
Isolated CM was monitored for IL-6 levels using an ELISA Kit (PeproTech Co., UK) following the manufacturer’s guidelines.
3.12. Statistical Analysis
The normal distribution of data was determined by the Kolmogorov-Smirnov test. Subsequently, the data were analyzed by one-way ANOVA and Dennett’s post hoc test, reported as means ± SD. The level of significance was set at P values of less than 0.05.
4. Results
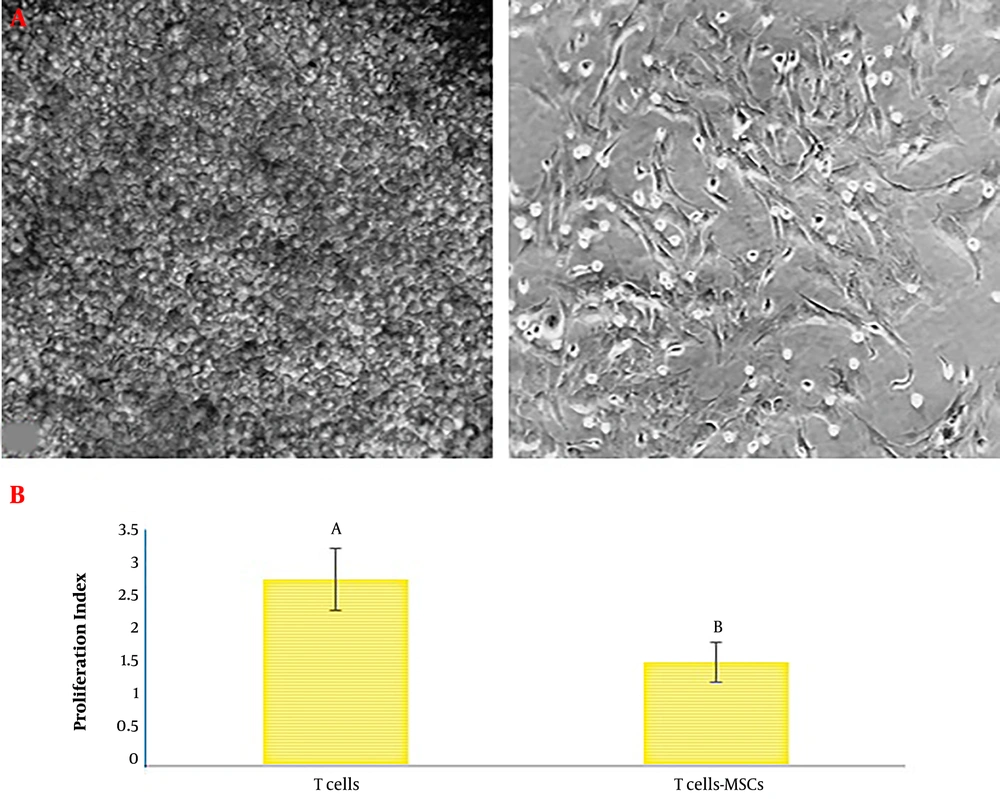
The test statistics are shown in Table 1. Based on the results, the alternative hypothesis was rejected. Therefore, we conclude that the findings came from a normal distribution (Table 1). Cells isolated from the bone marrow gently illustrated homogeneous fibroblast-like spindle-shaped cells (Figure 1A). Flow cytometric values exhibited that CD45 (a marker for hematopoietic cells) was absent in passage three (1.82% ± 0.34) whereas CD290 and CD29 (consensus markers for MSCs) were highly expressed (98.45% ± 0.57 and 95.81% ± 0.73, respectively). The suppression of poly-clonal T cell proliferation is one of the main hallmarks of MSCs (2). The attained data indicated that sub-culture three of MSCs could inhibit the proliferation of polyclonally stimulated T lymphocytes (Figure 1B).
A, displayed fields illustrate bone marrow-derived MSCs in different stages of the passage. Left image: at first, cells in bone marrow aspiration demonstrated spherical morphology. Right image: passage three of cells showed morphology similar to fibroblast cells typical of MSCs. B, proliferation index of T cell: T lymphocytes were pulsed with or without phytohemagglutinin (PHA) in the proximity of MSCs (10 splenocytes to one MSC) or lack of either MSCs for five days (different letters indicate a significant difference at P values of less than 0.001).
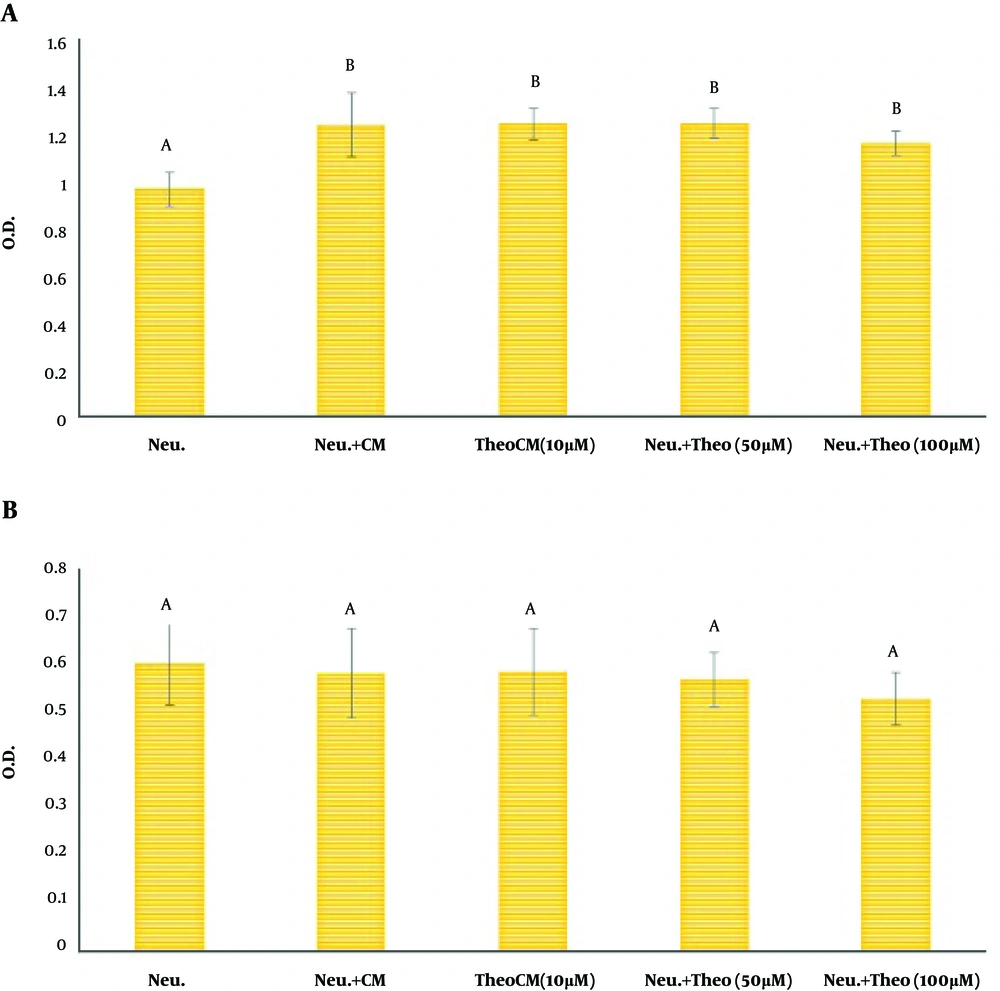
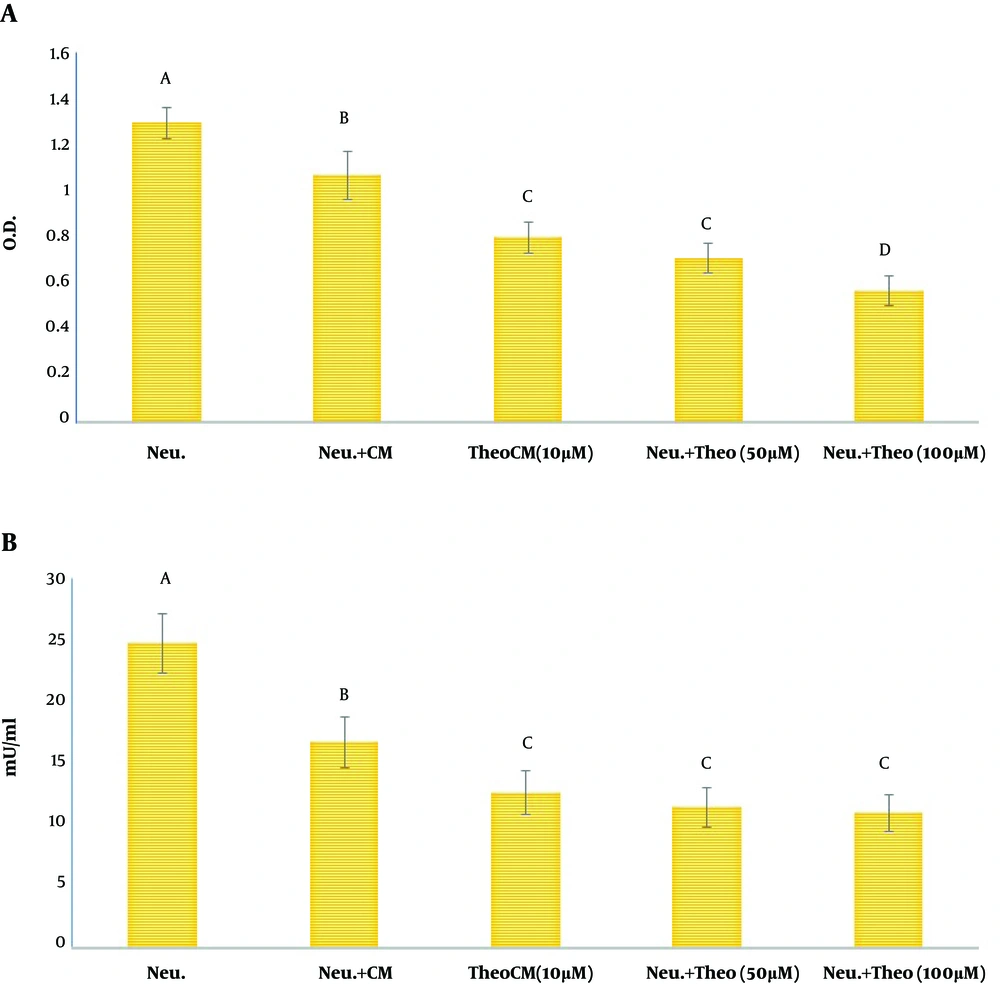
As exhibited in Figure 2A, the MTT reduction assay indicated that CM could significantly up-regulate the vitality of neutrophils (Figure 2A). Furthermore, CM isolated from MSCs pulsed with theobromine could not interfere with the protective effect of CM on co-cultured neutrophils. The NR engulfment by neutrophils did not illustrate any significant difference between neutrophils alone and neutrophils primed with CM without treatment or CM isolated from MSCs treated with theobromine (Figure 2B).
Assessment of neutrophils vitality by conditioned media of MSCs (CM) pulsed with different doses of theobromine. MSCs were co-cultured with different concentrations of theobromine (0 (control), 10, 50, and 100 µM) for 48 h. Then, CM was removed and co-cultured with neutrophils for 4 h. A, evaluation of MTT reduction assay by neutrophils; B, evaluation of neutral red uptake by neutrophils. Data are shown as mean ± SD. Neu: neutrophils; CM: conditioned medium; TheoCM: CM derived from MSCs pulsed with theobromine (different letters indicate a significant difference at P values of less than 0.01).
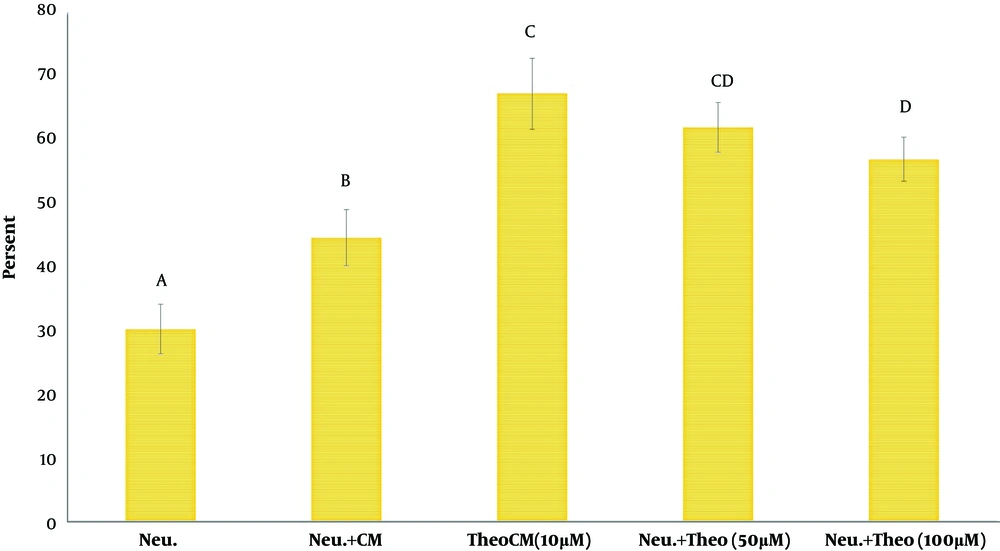
The findings showed that the phagocytic activity of neutrophils co-cultured with CM isolated from MSCs pulsed with theobromine or CM alone was significantly higher than the phagocytic activity of neutrophils alone (Figure 3). Data also suggested that the phagocytic ability of neutrophils cultured with CM isolated from MSCs pulsed with theobromine was significantly more outstanding than phagocytosis shown by neutrophils pulsed with CM alone (Figure 3).
Assessment of phagocytosis potential of yeast by neutrophils, co-cultured CM pulsed with different doses of theobromine. Values are presented as mean ± SD. Neu: neutrophils; CM: conditioned medium; TheoCM: CM derived from MSCs pulsed with theobromine (different letters indicate a significant difference at P values of less than 0.05).
To estimate the potential of ROS generation by neutrophils, the NBT reduction test was performed (35). Accordingly, the respiratory burst of neutrophils was markedly lower in neutrophils primed with CM isolated from MSCs cultured with theobromine or without theobromine than that of un-treated neutrophils (Figure 4A). However, this reduction in the respiratory burst was more remarkable in the neutrophil population co-cultured with theobromine than the reduction reported after the co-culture of neutrophils and CM alone (Figure 4A). As exhibited in Figure 4B, after co-culture with CM, n neutrophil treated with CM released MPO activity showed a significant decrease compared to neutrophils alone. Furthermore, CM isolated from MSCs pulsed with of theobromine significantly reduced the released MPO activity from neutrophil. compared to the MPO activity in the supernatant of neutrophils co-cultured with CM from MSCs that were not pulsed with theobromine (Figure 4B).
A, modulation of neutrophils respiratory burst; B, evaluation of MPO activity in the supernatant of neutrophils. Neutrophils were cultured alone or with CM for 4 h and then, were pulsed with phorbol 12-myristate 13-acetate (100 ng/mL) for 20 minutes. The results are presented as mean ± SD. Neu: neutrophils; CM: conditioned medium; TheoCM: CM derived from MSCs pulsed with theobromine (different letters indicate a significant difference at P values of less than 0.01).
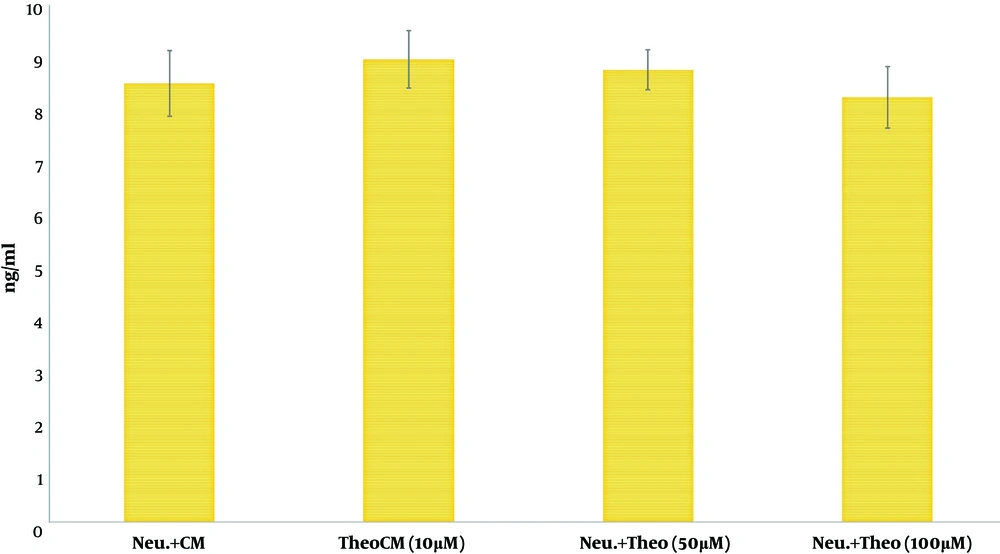
Finally, the gained data indicated no significant difference in the level of IL-6 between different CM sources isolated from MSCs alone or MSCs pulsed with different concentrations of theobromine (Figure 5).
5. Discussion
The in vitro results showed that theobromine upregulated osteogenesis and simultaneously downregulated adipogenesis by MSCs (36). Therefore, changes in other functions of stem cells by theobromine, such as their interaction with immune cells, are plausible. In this regard, MSCs render adenosine and respond adenosine receptors (A1R, A2AR, A2BR, and A3R), indicating that adenosine has a paracrine or autocrine function in the proliferation and differentiation of MSCs (18). Moreover, phosphodiesterase inhibition is a promising strategy to promote MSC survival and proliferation in damaged tissues like the infarcted heart (37). The phosphodiesterase inhibition can provide cGMP/PKG-I activity and promote the longer protection of MSCs against starvation or H2O2-induced oxidative damage (8). There are no or incomplete data concerning the role of theobromine in the potential fluctuation of the interaction between CM derived from MSCs and the neutrophil population in the inflammatory situation in this investigation. Thus, we inspected the consequence of CM derived from MSCs after treatment with theobromine on some important duty functions of neutrophil.
Interestingly, a new document indicated that the conditioned medium of MSCs treated with caffeine, another analog of methylxanthine, promoted the instruction of anti-inflammatory macrophages (18). Also, MSCs primed with caffeine at low to moderate concentrations (0.1 and 0.5 µM) could preserve the potential of neutral red ingestion by neutrophils and appointed the MSCs’ potential to protect neutrophils from apoptotic death. Nonetheless, caffeine at a high concentration (100 µM) intervenes with some schema of the mutual communication between MSCs and neutrophils (23).
Neutrophils have a short lifespan and therefore, their homeostasis and turnover should be highly regulated (38). It was revealed that MSCs in tissues significantly increased the lifespan of neutrophils by protecting them from apoptosis (10, 23). The MTT reduction test is a simple and rapid way of evaluating cell vitality (23). In the present survey, the data showed that the vitality of neutrophils, primed with CM, was higher than their survival rate in the absence of CM. Earlier documents reported that MSCs or CM could downregulate the mitochondrial pro-apoptotic protein Bax in neutrophils via IL-6 production (39). Besides, CM derived from MSCs primed with theobromine could not interfere with the protective effect of CM on co-cultured neutrophils. Theobromine is one of the adenosine antagonists. Therefore, it is conceivable that theobromine dose not interfere with IL-6 secretion by MSCs. Our results on IL-6 support this hypothesis.
Neutral red engulfed and accumulated in the lysosome fraction of neutrophils according to the cell activity like cell membrane integrity and cell viability (40). Based on the gained data, the NR uptake test, similar to the MTT assay, did not indicate any significant change between neutrophils, neutrophils primed with CM, and neutrophils pulsed with theobromine. Phagocytosis is the original hallmark of neutrophils, which involves in the ingest of invaders, derbies, and apoptotic bodies (41). Based on our results, the phagocytic potential of neutrophils was significantly enhanced in neutrophils pulsed with CM compared to neutrophils alone. Nevertheless, it has been shown that CM of theobromine-treated MSCs caused a remarkable enhancement in the phagocytic potential of co-cultured neutrophils.
Reactive Oxygen Substances are one of the main agents participating in the destruction of pathogens by neutrophils (35). However, when the generation of ROS is inappropriate or excessive, they partake in host tissue immunopathological conditions (42). Our data in this survey indicated that CM could markedly regress the ROS production by neutrophils. An earlier report also documents that the conditioned media of MSCs could suppress the basal and f-MLP-stimulated ROS generation by neutrophils (39). In this regard, our results also indicated that CM from theobromine-pulsed MSCs profoundly diminished the respiratory burst of co-cultured neutrophils more than did CM alone. On the other hand, MPO is a peroxidase enzyme, originally released by activated neutrophils. It has a pro-inflammatory property because of its oxidative nature (43). Similarly, CM from theobromine-pulsed MSCs profoundly decreased the level of MPO activity in co-cultured neutrophils more than did CM alone.
Multiple documents, similar to our results, exhibited that MSCs or their CM could change significantly the inflammatory function of neutrophils, like respiratory burst and MPO activity. (23, 24, 44). Interestingly, it has been proposed that similar to macrophages, migrating neutrophils are plastic and diverse and can possess different phenotypes depending on environmental factors (44). For example, it is suggested that there are tumor-associated macrophages may have to M1 and M2 phenotypes with anti-tumor and pro-tumor properties, respectively (44). Based on our results, it can be proposed that CM can promote neutrophils toward anti-inflammatory phenotypes. Moreover, theobromine exposure of MSCs can potentiate the formation of anti-inflammatory neutrophils. Higher phagocytic activity without the production of potentially harmful ROS promotes neutrophils to the resolute inflammatory reaction. Of note, no change in vitality (MTT assay test an NR uptake test) of neutrophils after co-culture with theobromine-pulsed CM indicates that no change in vitality (MTT assay test an NR uptake test) of neutrophils after co-culture with theobromine-pulsed CM indicates that neutrophils instead dynamically polarized they simply suppressed. Interestingly, earlier works report that caffeine, another analog of methylxanthine, at concentrations relevant to normal human consumption possesses immunomodulatory and anti-inflammatory benefits (18). Here, we suggest that some of these benefits might be due to the impression of adenosine receptor blocking on the impact of MSCs on neutrophils.
5.1. Conclusions
Theobromine treatment of MSCs can develop the education of anti-inflammatory neutrophils by the conditioned medium of MSCs. Our results offer a novel horizon into mechanisms that establish the anti-inflammatory and immunomodulatory benefits of theobromine.