1. Background
Diabetes mellitus (DM) is the most important endocrine disorder which is a growing worldwide health problem and is characterized by hyperglycemia, hyperlipidemia. It is estimated that by 2030, 366 million people will be suffering from DM (1-3). DM falls into type I and type II. This endocrine disorder is an essential foundation of a lot of chronic illnesses and it is an important cause of premature death (4). The prevalence and occurrence of diabetes are rising in most populations especially in developing countries (5). DM seems to increase oxidative stress with higher reactive oxygen production in seminal fluid and lipoperoxidation, sperm DNA fragmentation, sperm mitochondrial bioenergy alteration, enzymatic glycation end- products, decreased sperm count, motility and normal morphology (6, 7). Many herbal plants have been described to be beneficial in controling DM and improving its damages (8, 9).
Today, medicinal plants have attracted attention and consumers prefer to use them; moreover, they are easily available, inexpensive, and used in treatment of a variety of diseases (10). Plant products are frequently considered to be less poisonous and freer from side effects than synthetic ones (11) and have antioxidant and antimicrobial activities (12, 13). The World Health Organization (WHO) in its 31st meeting suggested the administration of medicinal herbs in developed countries. In this congress, WHO reported that medicinal herbs have standards compensation and healthiness (14). Selles et al. in 2013 reported that essential oils which are obtained from the aerial parts of Anacyclus pyrethrum (AP) leaves showed an antimicrobial activity (15). Moreover, Hydro-alcoholic extract of AP root showed a significant protection against memory deficit and oxidative stress in mice (16). Lalitha and Kumar (17) reported that alcoholic extract of AP root, at high concentration, had significant α-amylase inhibitory and good therapeutic effects for the management of diabetes and its damages. AP root extract in traditional medicine has been widely used for rejuvenation and vitality and in both male and female rats improved sexual behaviors (18). Bioactive phytochemicals in AP roots extract constituents such as phenol, flavonoids, alkaloids and tannins, have antioxidant activity that prevents oxidative tissue damage induced by free radicals and reactive oxygen species and thus may prevent a variety of diseases (19). Sharma et al. (20) reported that oral administration of AP root extract affected the sex hormones, sperm quantities and qualities in normal male rats. The aqueous extract of AP root increased the sperm count, fructose level in seminal vesicle, mounts frequency with fertility enhancing and reduced mount latency successfully in male albino rats. Studies revealed that Sertoli cells in diabetic male rats were impaired and the serum level of testosterone decreased compared to control group (21-23).
2. Objectives
Since existing literature indicated that AP root extract has androgenic effects on normal male rats, this survey was designed to evaluate the AP alcoholic root extract on FSH, LH, testosterone, sperm count and weight gain in the streptozotocin (STZ) induced diabetic male rats.
3. Methods
3.1. Animals
This experimental study was conducted on 60 Wistar-Albino adult (5 - 7 months old and 200 - 250 g) male rats. Each rat was weighed and placed in a cage separately. Animals were maintained in a controlled environment at a temperature of 22 ± 2°C and 12 h light/darkness cycle, with a fixed 12 h artificial light period (timer model: SUL180a, AC220V. China, 6 am to 6 pm). The rats had a standard animal chow; food and water were provided ad-libitum.
3.2. Experimental Design
Sixty rats were acclimatized for 7 days and divided into 6 groups (n = 10/each) (24) as follows randomly:
Control (C): which received animals chow and tap water for 4 weeks (trial period).
Diabetic control (DC): Which were made diabetic by using 60mg/kg STZ and received animal chow but did not receive any agents during trial period (24, 25).
Placebo group (P), which were diabetic and received dimethyl sulfoxide (solvent of alcoholic extract of AP root) during trial period.
Diabetic groups (DA1, DA2 and DA3) which received 50, 100 and 150 mg/kg alcoholic extract of AP root daily for a period of 4 weeks (trial period), respectively.
This animal study was carried out in accordance with recommendations from the Declaration of Helsinki and internationally accepted principle for the use of experimental animals, and they received institutional ethical approval from the Committee for Animal Research of Zahedan University of Medical Sciences (6541).
3.3. Preparation of the Alcoholic Extracts
Dry AP root was purchased from a herbal store in Saravan (a city located in southeast of Iran) and was then investigated in the department of biology in the Faculty of Science, Sistan and Baluchestan University, Zahedan, Iran. The AP root was powdered. Extraction was made by addition of 20 g powder in 80 mL of ethylic alcohol (96%) and left to sitfor 24 hours. The extract was filtered through a gauze cloth followed by filtration during a normal filter paper Watman No.1 (26). The product was a dark brown alcoholic extract which afterward was dried in incubator for 1 day at 45°C.
3.4. Induction of Diabetes
Type I diabetes was induced in overnight fasted male rat groups (DC, P, DA1, DA2 and DA3) by a single intra-peritoneal injection of STZ, at a dose of 60mg/kg. STZ was purchased from Sigma Corporation and before using was immediately dissolved in citrate buffer (pH = 4.5) (27). All of the test animals were diabetic and showed diabetic symptoms by polydipsia, polyuria, and polyphagia. After 5 days, the diabetic condition was found to be established when fasting blood sugar (FBS) in tail vein blood samples was higher than 226 mg/dL (28).
3.5. Collection of Blood Samples
At the end of trial period, under an overnight fasting period (12 - 14 hour), animals were anesthetized under insightful diethyl ether (Merck Germany), sacrificed and blood samples were collected from cervical vessels. All blood samples were collected in ordinary vials and centrifuged at 3000 rpm for 10 minutes to separate serum. Serum was removed (BH-1200 type Iran) and accumulated at -70°C for further analyses. At once, after obtaining blood samples, right and left vas deferens, epididymis, and testis were removed and weighed. Vas deferens and epididymis were dissected, cut and diluted with normal saline. Spermatozoa were counted using a hemocytometer and the count was justified per gram of vas deferens and epididymis (28).
3.6. Biochemical Analysis
Serum FSH and LH were determined by a sensitive rat kit (Cusabio Biotech Co. LTD), using double antibody enzyme-linked immunosorbent assay (ELISA), and serum testosterone was measured using standard laboratory (Mino bine human kit USA) methods.
3.7. Statistical Tests
Data were analyzed by SPSS V.11 software (SPSS Inc., Chicago, IL, USA) employing ANOVA followed by the Tukey testes. Results were expressed as mean ± SD. Statistical significance was accepted when the P value was lower than 0.05.
4. Results
4.1. The Effects of AP Root Alcoholic Extract on Serum FSH, LH and Testosterone
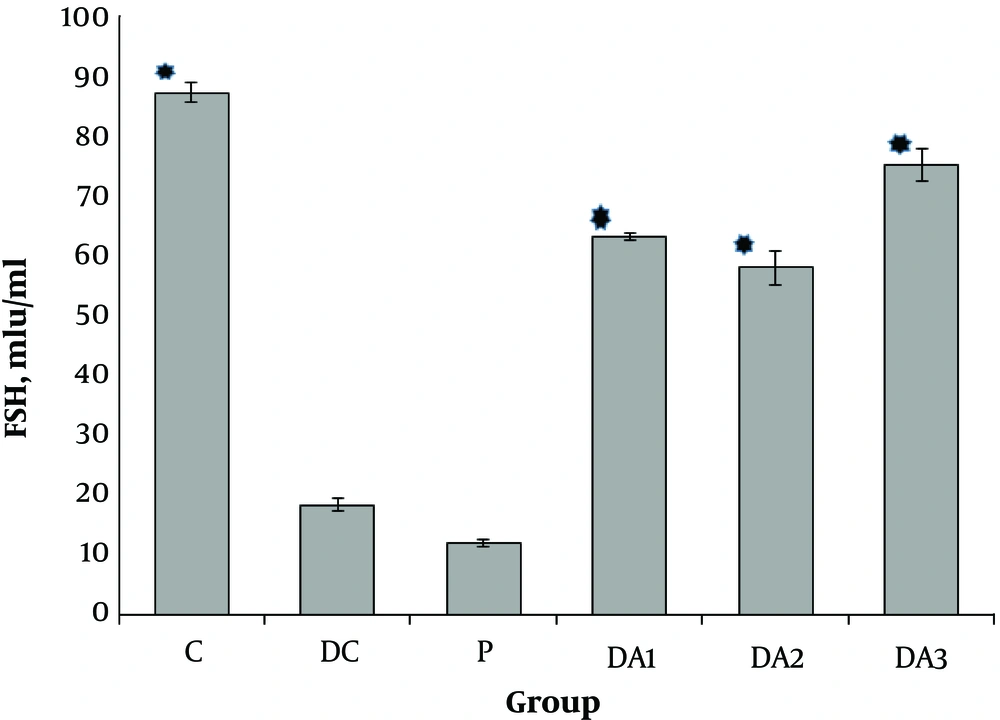
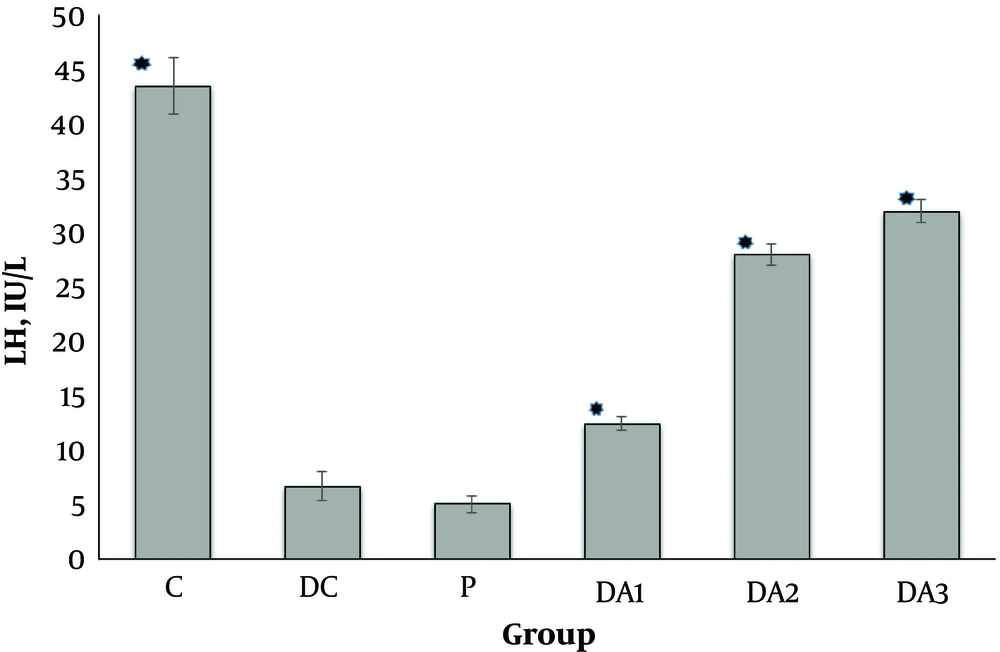
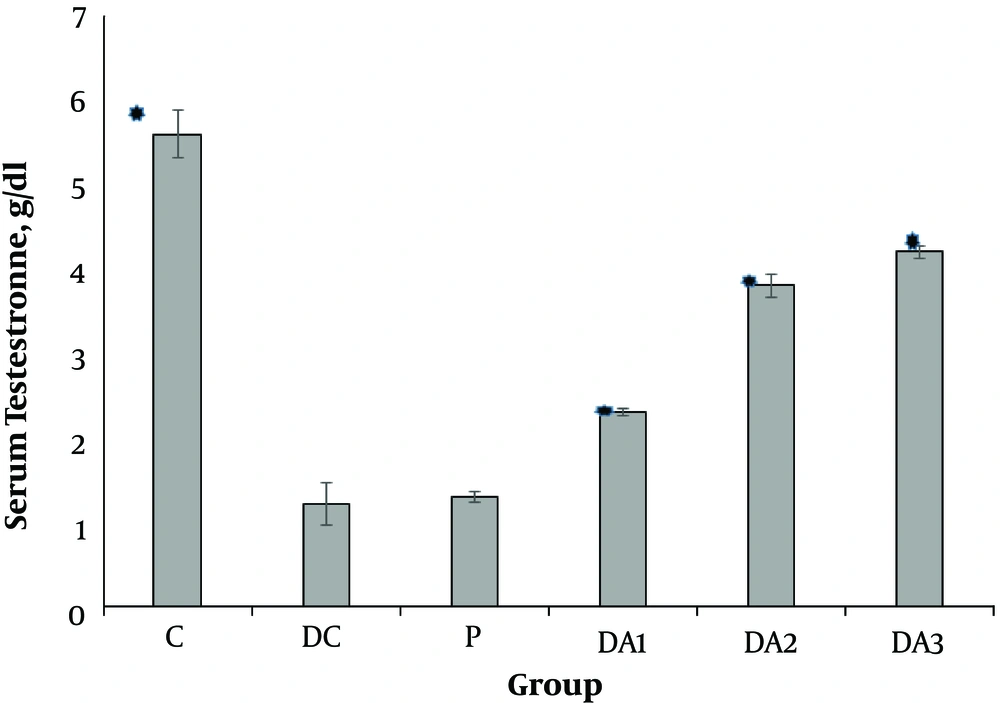
Our results indicated that serum FSH (Figure 1), LH (Figure 2) and testosterone concentration (Figure 3) in diabetic control group (DC) and placebo group (P) significantly decreased compared to control group (C). These values in all diabetic groups (DA1, DA2 and DA3) which received 50,100 and 150 mg/kg AP root alcoholic extract respectively, significantly increased compared to the DC and P groups ( location of Figures 1-3).
Mean (± SE) of serum Testestron concentration in different male rat groups based on treatment. * shows significant difference each group versus P group at the level of P < 0.01. Abbreviations: C, control group; DA1, DA2 and DA3, diabetic groups; DC, diabetic control group; P, placebo group.
4.2. The Effects of AP Root Alcoholic Extract on Sperm Count Per Gram of Epididymis and Vas Deferens
The results showed that sperm count per gram of epididymis and vas deferens tissues declined in the group DC and P compared to the group C (Table 1). In the diabetic groups which received AP root alcoholic extract (50, 100 and 150mg/kg), although sperm count per gram of epididymis showed an increase compared to the DC and P groups, statistical significance was observed only among group DA2 and DA3 against DC and P groups.
| Group | R Epididymis, N | L Epididymis, N | R Vas Deferens, N | L Vas Deferens, N |
|---|---|---|---|---|
| C | 36999.36 ± 612.65 A | 37606.6 ± 411.39 A | 27620.07 ± 660.44 A | 25659.37 ± 723.41 A |
| DC | 17943.69 ± 1133.22 B | 18185.68 ± 731.32 B | 20288.59 ± 1279.43 B | 17570.17 ± 310.75 B |
| P | 16675.47 ± 566.77 B | 16143.00 ± 1551 B | 18044.51 ± 836.07 B | 17246.60 ± 788.29 B |
| DA1 | 26018.92 ± 974.36 BC | 25119.40 ± 490.82 C | 21819.44 ± 1117.72 B | 23847.57 ± 906.01 A |
| DA2 | 28542.17 ± 940.82 C | 30325.00 ± 948.10 D | 26700.05 ± 809.17 A | 24435.45 ± 518.67 A |
| DA3 | 32426.75 ± 2117.08 AC | 30963.72 ± 1089.73 D | 31863.79 ± 14.28.48 A | 27040.92 ± 1181.64 A |
Abbreviations: C, control group; DA1, DA2 and DA3, diabetic groups; DC, diabetic control group; L, left; P, placebo group; R, right.
a Values within each column with different letters are significantly different (P < 0.05).
4.3. The Effects of AP Root Alcoholic Extract in the Epididymis Weight, Vas Deferens Weight and Body Weight Gain
weight of epididymis and testis and body weight gain significantly decreased in the DC and P groups compared to the group C. These values in the DA2 and DA3 groups significantly increased compared to the DC and P groups. Tables 2 and 3 show significant or non-significant differences among all groups in variables of epididymis, vas deferens and testis (per gram tissue) weight and body weight gain.
| Group | R Epididymis, mg | L Epididymis, mg | R Vas Deferens, mg | L Vas Deferens, mg |
|---|---|---|---|---|
| C | 590 ± 9.17 A | 570 ± 3.78 A | 120 ± 1.58 A | 130 ± 1.27 A |
| DC | 380 ± 4.74 B | 390 ± 3.79 B | 70 ± 2.84 B | 82 ± 1.57 B |
| P | 380 ± 7.27 B | 370 ± 8.22 B | 60 ± 1.89 B | 80 ± 2.53 B |
| DA1 | 390 ± 8.86 B | 430 ± 6.32 C | 80 ± 2.53 C | 80 ± 1.89 B |
| DA2 | 510 ± 16.13 C | 510 ± 9.81 D | 90 ± 1.58 C | 90 ± 1.57 C |
| DA3 | 540 ± 6.96 AC | 520 ± 9.17 D | 90 ± 2.54 C | 100 ± 1.26 C |
Abbreviations: C, control group; DA1, DA2 and DA3, diabetic groups; DC, diabetic control group; L, left; P, placebo group; R, right.
a Values within each column with different letters are significantly different (P < 0.05).
| Group | R Testis, g | L Testis, g | Body Weight Gain, g |
|---|---|---|---|
| C | 1.33 ± 0.06 A | 1.32 ± 0.06 A | 262.7 ± 1.27 A |
| DC | 0.97 ± 0.13 B | 0.93 ± 0.09 B | 175.11 ± 1.92 B |
| P | 0.89 ± 0.26 B | 0.88 ± 0.02 B | 178.22 ± 1.7 B |
| DA1 | 1.03 ± 0.06 B | 1.06 ± 0.02 B | 184.4 ± 2.05 B |
| DA2 | 1.24 ± 0.02 A | 1.19 ± 0.01 A | 209.62 ± 2.31 C |
| DA3 | 1.29 ± 0.02 A | 1.25 ± 0.02 A | 214.5 ± 2.01 C |
Abbreviations: C, control group; DA1, DA2 and DA3, diabetic groups; DC, diabetic control group; L, left; P, placebo group; R, right.
a Values within each column with different letters are significantly different (P < 0.05).
5. Discussion
The results of this study showed that the alcoholic extract of AP root is helpful in reproductive hormones and organs in the diabetic male rats. In the present study, FSH, LH and testosterone serum concentration were significantly lower in the diabetic groups (DC and P groups) which did not receive AP root alcoholic extract. In the DA2 and DA3 groups which received 100 or 150 mg/kg alcoholic extract of AP root, respectively, FSH, LH and testosterone serum concentrations were significantly higher than DC and P groups. On the other hand, sperm count in epididymis and vas deferens per gram of tissues decreased significantly in DC and P groups compared to the control group. This shows that DM is corresponding to a reduction of testosterone impairment (29). These parameters increased by increasing concentration of AP root alcoholic extract (important in clinical settings) in diabetic male rat groups which received the extract of AP root against DC and P groups; however, this difference in group DA1 was not significant compared to DC and P groups. Our findings revealed that the same results existed in the epididymis weight too. Weight gain in DC and P groups was significantly lower than control group. The results showed that in the diabetic rats, weight gain increased by using AP root alcoholic extract especially in higher dose. The previous study showed that DM or using STZ to induce type1 diabetes (30), has an effective cause in sperm damage, disrupts seminal fluid release following gland infection or inflammation in semen , decreases sperm progressive motility, induces testicular dysfunction and failure by apoptotic death, disrupts seminiferous tubule structure, decreases spermatogenic cell series, causes atrophy of the tubules, brings about varying degrees of spermatogenetic arrest, causes erectile and/or ejaculatory dysfunction and decreases gonadotropin hormones concentration (7, 31-33). Moreover, other studies have indicated that diabetes induced oxidative stress and balance between the production of reactive oxygen species and the ability of deoxidation is disturbed with pathological effects on liver, pancreas, kidney, proteins and testicular DNA and reproductive organs. Vasculopathy is the most damage that produces microvascular complications in diabetes and insufficient blood supply is one of the major causes of testicular dysfunction (29, 34). It is well-known that some herbs have bioactive components which have a beneficial effect on human health (35). Flavonoids in plants are phytochemicals with biological and antioxidant activities, which is protective and prevent disease (36). Moreover, some studies have also shown that flavonoids have androgenic effects (18). Androgens not only have an essential role in prevention and treatment of gonadal dysfunction and in fertility (19) , but also increase the body weight and genital organs (29). Many studies in AP showed that it has some beneficial effects and can be used effectively for different clinical purposes such as antioxidant activity (37) antibacterial effect (38), anticonvulsant (39), antidiabetic (40), and reproductive activities (18).
Our findings also showed that in the diabetic control and placebo groups, serum FSH, LH, testosterone, and sperm count in epididymis and vas deferens decreased which can be attributed to diabetes (6, 7). In diabetic groups that received AP root alcoholic extract, reduction of these factors has been modified. It could be related to protective and androgenic effects of AP root extract. These findings are in line with findings by Sharma et al. (2012) that reported AP root ethanol extract had positive effects on male normal rat reproductive system by increasing sperm count, motility, serum FSH, LH and testosterone. LH and FSH stimulate the gonads to produce gonadotropin hormones. In testes, LH stimulates synthesis and secretion of testosterone by the Leydig cells. FSH is critical for sperm production since it supports the function of Sertoli cells, which, in turn, support many aspects of sperm cell maturation. Hence, decreasing in these hormones may disrupt reproductive functions and their organs. Previous studies demonstrated that oral administration of aqueous extract of AP root daily for 60 days at a dose level of 150 mg/kg caused restoration of reproductive hormones (T, LH and FSH). This finding of our study is in line with Shahraki et al. and Sharma et al. who reported that AP extract used in healthy adult male rats increased serum concentrations of mentioned reproductive hormones and produced testosterone-like effect (androgenic effect) by improving the fertility since it enhanced spermatogenesis and testis weight (20, 29). This may be due to the fact that AP root extract has many components such as alkyl amide and polymeric carbohydrate (20) and unknown components which can prevent the testosterone feedback effect on hypothalamus and adenohypophysis and can stimulate neuro-endocrine system (hypothalamus-hypothesis-testis) axis, and hormone release and showed increased serum FSH and LH concentration. This section of the present study is supported by Pahuja et al. (2013) who reported that AP extracts administration showed significant protection effects on hypothalamus nuclei and showed antiepileptic properties and neuro protective effects by decreasing oxidative stress in PTZ kindled mice (16, 41). Moreover, other reports showed that the extracts of AP can inhibit the porcine pancreatic amylase activity and has anti-hyperglycemic effects and can improve diabetes complications and reproductive dysfunctions (28, 42). The results obtained from present study revealed that administration of AP root alcoholic extract increased sperm count in epididymis and vas defrens per gram tissues, but this difference was not significant in DA1 group which received 50 mg/kg AP root alcoholic extract compared to the diabetic or placebo groups. These findings are in disagreement with finding of Sharma et al. (20). These differences might be due to differences of the dosage, age and seasonal administration of extract.
5.1. Conclusions
We concluded that although diabetes type I reduces sex hormone and increases damage to sex organs in the male rats, administration of the AP root alcoholic extract improves diabetic impaired tissues in diabetic rats and stimulates the hypothalamus-adenohypophysis- gonad axis and repairs reproductive dysfunctions especially in the 100 and 150 mg/kg AP root alcoholic extract.