1. Background
The increase of sedentary lifestyle is one of the growing health problems in different societies that covertly cause increasing the risk of chronic diseases, including non-alcoholic fatty liver disease (1). Fatty liver is one of the chronic liver disorders associated with lipid accumulation in hepatocytes (2). Hepatocellular carcinoma has been designated as a complication of non-alcoholic fatty liver disease (NAFLD), and this association has been supported by multiple small observational studies, which suggest age and advanced fibrosis as significant risk factors (3).
Extensive epidemiological studies show that over one billion people in the world are overweight, and at least 355 million people are clinically obese (4, 5). The prevalence of NAFLD increases by 70% to 80% in subjects with obesity (6). The prevalence of NAFLD in Iran is estimated to be between 21.5% and 31.5% (7). Thus, NAFLD is a highly significant and multi-factorial disease associated with important key modifiable risk factors, including obesity. The increased plasma concentration of aspartate aminotransferase (8) and alanine aminotransferase (9) cytoplasmic enzymes may be due to their increased synthesis and secretion or related to decreased catabolism. Considering the role of thyroid hormones in increasing the metabolic activities of body tissues, it is likely that the increase of these enzymes is the result of catabolism reduction (10). Hypothyroidism may be due to a problem with the thyroid gland or due to the disease in the pituitary gland and hypothalamus (11). Hypothyroidism has been identified as a non-alcoholic fatty liver development factor due to its important role in fat metabolism (12). Studies have shown that thyroid hormones affect almost all aspects of carbohydrate metabolism. Also, the triiodothyronine (T3) hormone increases the glucose output of the liver, promotes the GLUT2 glucose transporter, and provides a skeletal stimulant for GLUT4 glucose transporter (13). In hypothyroidism, insulin resistance is produced, and glucose is not transmitted to the muscle (14). The disruption of glucose metabolism and insulin resistance is a gradual process that begins with overweight and obesity. Aerobic exercise can increase insulin sensitivity (15).
Today, the objectives of body composition measurements have largely turned to general health (16, 17). One of the health assessment methods is the use of waist circumference (WC) and waist-to-hip ratio (WHR), which are known as proper indicators associated with diseases related to the distribution of lipids (18). The reduction of abdominal obesity can be an important benefit of exercise, which can significantly improve metabolic indices and decrease fatty liver and liver enzymes in fatty liver patients; along with increasing physical capabilities, it helps treat the disease (19).
2. Objectives
Therefore, this study aimed to investigate the effect of aerobic exercise on non-alcoholic fatty liver enzymes, thyroid hormones, and some anthropometric indices in obese children to provide an effective aerobic exercise as a non-medical treatment for NAFLD and hypothyroidism.
3. Methods
3.1. Subjects
The present quasi-experimental study was conducted on a field basis. The statistical population included all obese boy students with BMI above the 95th percentile based on the CDC growth curves (20) aged 9 - 12 from the primary schools of Darehshahr. We first explained the importance and nature of research to children and their parents. After obtaining informed consent from parents, 24 children were selected and randomly divided into two groups of control (12) and experiment (12) according to the study criteria. We included obese children with no exercise habits (≤ one session per week and ≤ 30 min per session) over the past year. We excluded subjects with adverse medical problems (measured based on medical interviews and resting electrocardiograms done by a medical doctor) and those who declined to participate in the current study. The diagnostic criteria of NAFLD were established by the diagnostic guidelines for NAFLD in the Asia-Pacific Region. Ultrasonography was then performed on their livers to determine the fatty liver level of each subject by grades I, II, and III. The anthropometric indices were also measured.
3.2. Training Program
Under the supervision of the researcher, subjects of the experimental group conducted four sessions of training (average 45 minutes) each week for 12 weeks while the control group engaged in their daily activities without intervention. The training method was constant in all the sessions, including warming up, stretching, and active cooling down. To prevent training compatibility, we started with running for 15 minutes at the intensity of 50% of maximum oxygen consumption in the first week, which gradually increased to the intensity of 70% for 25 minutes at the end of the eighth week and continued until the end of the 12th week (Table 1).
| Movement Type | Duration, min |
|---|---|
| Warming up | 10 |
| Stretching | 5 |
| Running at the intensity of 50% - 70% of VO2max | 20 |
| Active cooling down | 10 |
3.3. Blood Sampling
Blood samples were taken from subjects in two stages before and after the training program. Before beginning the training program and in the pretest phase, a laboratory specialist took 10 mL blood from the subjects’ antecubital fossa veins in one day after 10 - 12 hours of fasting and about 72 hours before the first training session. All evaluations were carried out in the same conditions (7 - 9 AM at 26°C - 28°C). For serum isolating, blood samples were centrifuged for 15 minutes at 4°C and 3,000 rpm. Then, ALT, AST, and ALP liver enzymes were measured by α-ketoglutarate reaction using Pars Test Laboratory Kits (Iran). The levels of thyroid hormones (T3), thyroxine (T4), and thyroid stimulating hormone (TSH) were determined by the ELISA method using Autobio Kit (China). Finally, 48 hours after the last training session, the second stage of blood sampling was done for all subjects in the same conditions.
3.4. Liver Ultrasonography
To assess the effect of training on the degree of non-alcoholic fatty liver and the percentage of fat in the liver, all of the subjects underwent ultrasonography of the liver before and after training at 8 - 10 AM. Ultrasonography of the liver was performed by an expert gastroenterologist using a point-of-care ultrasound device (general electric (GE) ultrasound device, USA), and the level of fatty liver of each subject was specified by grades I, II, or III (0 = none, 1 = mild, 2 = moderate, 3 = severe). A mild fatty liver was recognized by a slight increase in liver echogenicity and relative preservation of echoes from the walls of the portal vein. A moderate fatty liver was recognized by the moderate loss of echoes from the walls of the portal vein, particularly from the peripheral branches, and moderate diffuse abnormally bright echoes. A severe fatty liver was recognized by a greater reduction in beam penetration, the loss of echoes from most of the portal vein walls, and extensive abnormally bright echoes (21). Ultrasound image acquisition and data interpretation were performed by a research team member blinded to treatment allocation.
3.5. Estimating Anthropometric Indices
Before and after the training program, anthropometric indices, including WC, waist-to-weight ratio, WHR, and BMI, were estimated using Lufkin strip with a precision of one millimeter (made in America) and the SECA height meter with a precision of one millimeter (made in Germany). Estimating the anthropometric indices in this study was based on the international standards of anthropometrics measurement by two anthropometrics with third-degree international certification.
3.6. Statistical Analysis
Data were analyzed by SPSS version 22 software. Descriptive statistics were used to determine the mean, standard deviation, minimum, and maximum values of data. According to the Kolmogorov-Smirnov test, data showed normal distribution. Therefore, parametric statistics were used. Then, for studying the significant differences in means, repeated-measures ANOVA was used, followed by Bonferroni post hoc test at a level of P < 0.05.
4. Results
There were no statistically significant differences in body mass index, height, weight, age, liver enzymes (AST, ALT, and ALP), and thyroid hormones (T3, T4, and TSH) between the groups at the beginning of the study and the groups were homogeneous (Table 2).
| Group | ||
|---|---|---|
| Variable | Experimental Group | Control Group |
| Age, y | 10.33 ± 0.89 | 11 ± 1.28 |
| Weight, kg | 63.75 ± 2.42 | 64.17 ± 2.59 |
| Height, cm | 150.67 ± 2.64 | 151.58 ± 2.71 |
| Body mass index, kg/m2 | 28.06 ± 0.47 | 27.91 ± 0.67 |
aValues are expressed as mean ± SD.
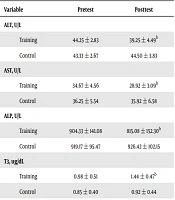
After 12 weeks of aerobic training, the levels of liver enzymes (ALT, ALP, and AST), thyroid hormones (TSH and T4), and anthropometric indices (BMI and WC) were significantly lower in the training group than in the control group (P = 0.002, P = 0.047, P = 0.003, P = 0.023, P = 0.002, P = 0.001, and P = 0.033, respectively). Also, the T3 hormone level was significantly higher in the training group than in the control group (P = 0.011). Although mean WHR was lower in the experimental group, it was not statistically significant (P = 0.228) (Table 3). Moreover, the 12 weeks of aerobic training significantly decreased the degree of NAFLD (Tables 4 and 5).
| Variable | Pretest | Posttest |
|---|---|---|
| ALT, U/L | ||
| Training | 44.25 ± 2.83 | 39.25 ± 4.49b |
| Control | 43.33 ± 2.67 | 44.50 ± 3.83 |
| AST, U/L | ||
| Training | 34.67 ± 4.56 | 28.92 ± 3.09b |
| Control | 36.25 ± 5.54 | 35.92 ± 6.58 |
| ALP, U/L | ||
| Training | 904.33 ± 141.08 | 815.08 ± 152.30b |
| Control | 919.17 ± 95.47 | 926.42 ± 102.15 |
| T3, ug/dL | ||
| Training | 0.98 ± 0.51 | 1.44 ± 0.47b |
| Control | 0.85 ± 0.40 | 0.92 ± 0.44 |
| T4, ug/dL | ||
| Training | 10.27 ± 1.5 | 8.70 ± 0.43 |
| Control | 10.83 ± 2.16 | 10.45 ± 1.71 |
| TSH, mu/L | ||
| Training | 3.15 ± 1.20 | 2.62 ± 0.97b |
| Control | 3.89 ± 1.19 | 3.64 ± 1.06 |
| WHR | ||
| Training | 0.89 ± 0.034 | 0.86 ± 0.04 |
| Control | 0.88 ± 0.041 | 0.88 ± 0.04 |
| WC, cm | ||
| Training | 80.92 ± 5 | 76.83 ± 4.32b |
| Control | 79.95 ± 3.34 | 80.21 ± 3.31 |
| BMI, kg/m2 | ||
| Training | 28.06 ± 0.47 | 26.97 ± 0.75b |
| Control | 27.91 ± 0.67 | 27.99 ± 0.75 |
aValues are expressed as mean ± SD.
bSignificat.
| Group | Healthy | Grade I | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Experimental | 0 (0) | 8 (66.67) | 4 (33.34) | 0 (0) |
| Control | 0 (0) | 7 (58.34) | 5 (41.67) | 0 (0) |
aValues are expressed as No. (%).
| Group | Healthy | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Experimental | 8 (66.67) | 3 (25) | 1 (8.33) | 0 (0) |
| Control | 0 (0) | 7 (58.34) | 5 (41.67) | 0 (0) |
aValues are expressed as No. (%).
5. Discussion
Based on the results of this study, aerobic training for four days a week over 12 weeks could decrease liver enzymes (AST, ALT, and ALP) and thyroid hormones (TSH and T4), increase thyroid hormone T3, and improve anthropometric indices including BMI and WC. Also, it significantly decreased the degree of NAFLD. At the end of the study, 66.67% of the participants in the experimental group were healthy (Table 5) while before exercise, 33.34% had grade II fatty liver, and the rest had grade I fatty liver (Table 4). The new finding of this study was that this particular type of exercise could be effective in the treatment of NAFLD, hypothyroidism, and anthropometric indicators in obese children. The findings of this study are consistent with the study results of Da Silva (22), Reddy and Rao (23), and Clarkson (24) regarding the effectiveness of aerobic training in the fatty liver condition. In a study of 105 patients with high levels of liver enzymes, Khosravi et al. (25) reported a significant positive correlation between liver echogenicity and ALT and AST enzyme levels. In this study, the ALT enzyme was associated not only with the degree of fat accumulation in the liver, indicating the role of abdominal fat as a predictor of higher levels of this enzyme in NAFLD.
In a cross-sectional study to compare the physical activity of people with fatty liver and healthy people between 2003 and 2011, the level of physical activity in patients with non-alcoholic steatohepatitis was lower than that of the control group and only 8.53% of the patients affected. On the other hand, 6.83% of participants in the control group had physical activity according to the recommendations (22). Physical training has also shown to increase the basal metabolic rate (BMR) (25). Another study showed that the prevalence of diagnosed NAFLD and abnormal levels of ALT increased steadily with increased degrees of hypothyroidism (26). In this study, the decreased levels of liver enzymes were associated with improvements in hypothyroidism. Also, the results of this study showed that 12 weeks of aerobic training significantly decreased NAFLD. As exercise and physical activity can increase the oxidation of fats, the accumulation of fat in the liver is prevented.
Daily energy consumption increases in aerobic training and leads to an increased rate of lipid oxidation in muscles and mitochondria. Therefore, visceral obesity is reduced (19). Furthermore, aerobic training increases insulin sensitivity in lipid tissue and can increase fatty acids in the liver. As a result, aerobic training reduces the transport of free fatty acids to the liver and prevents lipid deposition in the liver (8). Before aerobic training, 67.66% of the participants in the experimental group had grade I fatty liver, and 34.33% had grade II fatty liver. In the end, the rates of grade I and II fatty liver disease were 25% and 33.8%, respectively, and 67% of the participants were healthy. Therefore, aerobic exercise could increase insulin sensitivity in adipose tissue and enhance the oxidation of fatty acids in the liver. By reducing fat deposits in the liver, fatty liver disease improves, and liver enzymes return to normal levels.
The T4 hormone accounts for about 95% of hormones secreted from the thyroid gland. Although the level of T3 secretion from the thyroid gland is negligible, it plays a major role in the body. Most of T4 in the blood results from the conversion of T3 to T4 in peripheral tissues such as the liver and kidneys. Generally, 80% of T3 is found in the blood and in the liver and 20% in the thyroid (27). The results of this study showed an increase in T3 and a decrease in T4. Aerobic training is likely to increase the rate of conversion of T3 to T4. We recommend the regular measurement of weight and waist circumference as indicators of obesity for this age group. These measurements help the person to be aware of the risk earlier and begin timely treatment. The researcher recommends all obese children with NAFLD to do aerobic training for treating and managing the disease. It also suggests that children with hypothyroidism do aerobic training for treating their disease.
5.1. Conclusions
This study showed that 12 weeks of aerobic training significantly reduced the level of liver enzymes (AST, ALT, and ALP) and thyroid hormones (TSH and T4) in obese children with NAFLD. Also, after 12 weeks of training, several key anthropometric indices (waist circumference and body mass index) were significantly lower in the training group than in the control group, and the thyroid T3 hormone level was significantly higher. However, although the WHR anthropometric index was lower in the training group than in the control group, this was not significant. The researcher suggests that all obese children with NAFLD do aerobic training for treating and managing the disease. It also suggests that children with hypothyroidism do aerobic training for treating their disease.