1. Background
Strenuous exercise training is associated with increased oxygen uptake (1). The oxygen consumption by cells and organelles such as mitochondria increases following exercise training and leads to the production of reactive oxygen species (ROS) (2). Production of ROS beyond the endogenous antioxidant defense system induces oxidative stress and results in the destruction of cell structures such as nucleic acids, proteins, and lipids (3). Malondialdehyde (MDA) is generated by the breakdown of the oxidation reactions of polyunsaturated fatty acids and thus is considered as a valid marker of oxidative stress (4). Delayed gastric emptying, gastrointestinal tract inflammation, and gastric ulcer disease occur during exercise, especially following strenuous events such as moderate- to high-intensity long-distance running (5-7). These symptoms are caused by different factors, such as the underlying mechanism that increases the production of oxygen-derived free radicals (8).
During exercise, there is a blood shunting to the skin and muscles at the expense of the gastrointestinal system. According to evidence, in humans exercising at 70% of VO2max, a 60% - 70% decrease is observed in splanchnic blood flow (9). Antioxidant enzymes provide the strongest antioxidant defense, although all antioxidants are important to proper neutralization of oxidative stress (10). The most important antioxidant enzyme is superoxide dismutase (SOD), which neutralizes dangerous superoxide anion radicals by converting it to hydrogen peroxide (11). SOD contains metal ions that battle with the metal-induced toxic effects of free radicals (12).
Endurance athletes frequently experience exercise-induced gastric symptoms (13, 14) and running events lead to a higher incidence of such problems compared with other types of training such as cycling and swimming (15, 16). Many researchers studied the potential benefits of antioxidants supplementation during endurance training to maintain optimal antioxidant status in exercising individuals (17). It is shown that turmeric exerts anti-inflammatory, anti-cancer, antioxidant, wound healing, and anti-microbial effects and plays a role in treating liver and digestive problems and reducing serum cholesterol levels (18). The yellow pigments known as curcumin are active ingredients comprising 2% - 5% turmeric and have antioxidant properties similar to vitamins C and E (12). It is suggested that curcumin activates multifactorial mechanisms that protect the stomach. According to studies, curcumin scavenges ROS and inhibits gastric peroxidase activity (19).
2. Objectives
Recently, attempts to find supplements for overcoming gastric ulcers induced by strenuous endurance training and the whole body oxidant status are increasing. Therefore, the current study aimed at investigating the effects of curcumin supplementation during nine weeks of endurance training on the gastric antioxidant capacity (SOD) and lipid peroxidation (MDA) of serum in male Wistar rats.
3. Methods
3.1. Animals
In the current applied study, 26 male Wistar rats aged nine weeks and weighed 215.87 ± 20.49 g were provided by the Pasteur Institute, Iran. All animals were housed in polycarbonate cages (four rats in each cage) and maintained in a 12:12 hour light/dark cycle at 22 ± 1.4ºC and 40% - 50% humidity in a controlled room. Water and food were available for rats ad libitum.
3.2. Experimental Design
After one week of acclimation to the training protocol, rats were randomly assigned based on their weights to four groups: the control (n = 6), curcumin (n = 6), endurance (n = 7), and endurance + curcumin (n = 7) (Table 1).
| Number | Group | N | Weight at Baseline (g) | Weight After the Intervention (g) | Weight Change (g) | Weight Change (%) |
|---|---|---|---|---|---|---|
| 1 | Control | 6 | 220.14 ± 15.11 | 323.1 ± 20.18 | +102.96 | +46 |
| 2 | Curcumin | 6 | 216.70 ± 26.21 | 295.2 ± 43.11 | +78.5 | +36 |
| 3 | Endurance | 7 | 211.22 ± 18.9 | 245.21 ± 19.6 | +33.99 | +16 |
| 4 | Endurance + curcumin | 7 | 215.45 ± 21.75 | 246.44 ± 25.54 | +30.99 | +14 |
Endurance training protocol: The endurance training protocol consisted of nine weeks (five sessions per week) of running (20) on a motorized rodent treadmill (Pishro Andishe Sanat Co., Iran). The training program started at a speed of 10 m/minute for 30 minutes in the first week and increased incrementally (every week) up to 35 m/minute for 70 minutes with 80% - 85% of VO2max in the last week (Table 2). Animals were weighed before each training session and to prevent overtraining, an under-loading week was taken at the 5th week. Before starting each training session, rats performed five minutes of warming-up at a speed of 8 m/minute and five minutes of cooling-down at a speed of 6 m/minute at the end of the exercise. The control group (sham group) underwent interventions similar to those of the treatment groups (etiolate solvent injection, etc.), except running and curcumin supplementation. The training protocol was conducted in the animal house of the University of Zanjan.
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Duration (min) | 30 | 40 | 45 | 50 | 35 | 60 | 70 | 70 | 70 |
| Speed (m/min) | 10 | 20 | 20 | 25 | 15 | 30 | 30 | 35 | 35 |
3.3. Curcumin Supplementation
For preparation, because of different qualities of turmeric rhizomes collected from different areas, 1 g of curcumin powder provided by Merck company (Germany) was mixed with 1 mL of alcohol (%96 ethanol) and then diluted with an etiolate solvent (Merck company, Germany) to reach a 100-mL solution. It was then administrated at 30 mg/kg of body weight (BW) intraperitoneally (IP) three days a week in the curcumin and endurance+curcumin groups (21).
3.4. Tissue Preparation
Forty-eight hours after the last training session and 24 hours fasting, the animals were anesthetized with ketamine (30 - 50 mg/kg of BW) and xylazine (3 - 5 mg/kg of BW) (22). Blood samples were taken from the hearts of the rats, and then the abdominal cavity was quickly opened to excise the stomach. Blood samples were centrifuged at 3000 rpm for 10 minutes at 4°C, and the collected sera were stored at -20°C until analysis. Tissue specimens were frozen immediately in liquid nitrogen (at -196°C) and stored at -80°C. Then, tissue specimens were homogenized with phosphate buffer saline (pH 6.5) and centrifuged at 9000 rpm for 20 minutes. The supernatants were used to measure the activity of SOD, and sera were used to measure MDA levels.
3.5. Measurement of SOD Activity
SOD activity was assayed by the spectrophotometric method introduced by Woolliams et al., using the SOD kit (rat SOD ELISA kit, Randox Company, UK) (23), and the results were expressed as U/mg of protein.
3.6. Serum MDA Measurement
Serum MDA levels were assessed utilizing the thiobarbituric acid reactive substances (TBARS), based on the method introduced by Kaya et al. The concentration of TBRAS was measured at 532 nm using a standard curve of MDA, and the results were expressed as nM of MDA/mg of protein (24).
3.7. Statistical Analysis
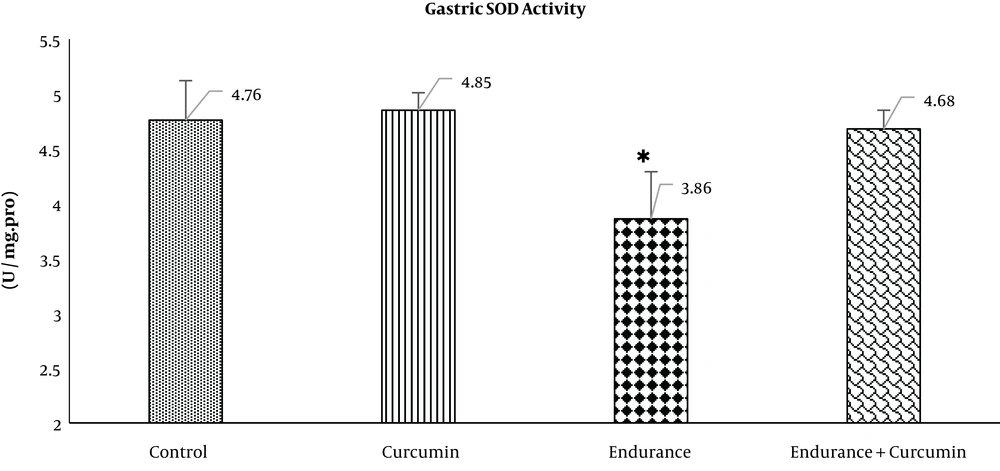
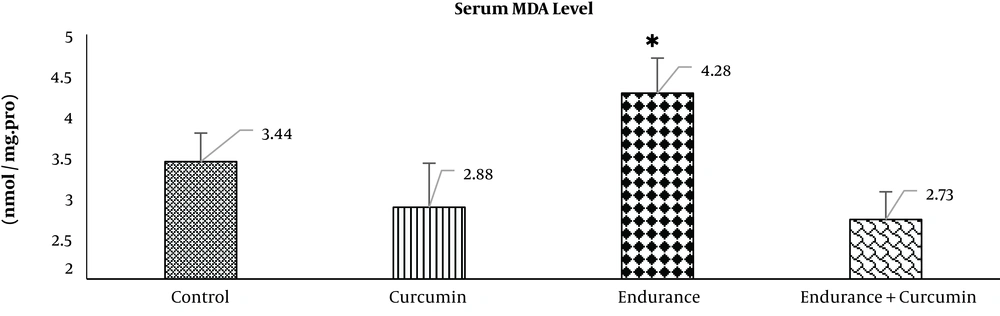
Data were expressed as mean ± standard deviation (SD). After ensuring the normality of data using the Shapiro-Wilk test, all data were analyzed using SPSS version 24.0. Gastric SOD and serum MDA levels were analyzed using the one-way analysis of variance (ANOVA), followed by the Tukey post hoc test for intergroup comparisons. P < 0.05 was considered as the level of significance. The results of data analysis are shown in Figures 1 and 2.
3.8. Ethical Considerations
The ethical guidelines approved by the Institutional Animal Ethics Committee of the University of Zanjan were followed, and laboratory conditions were maintained according to the university guidelines for caring (code no. ZNU.ECRA.2017-16).
4. Results
Based on the Shapiro-Wilk test results, the normality of the distribution of data for SOD activity was normal: the control (0.88), curcumin (0.2), endurance (0.55), and endurance + curcumin (0.47) groups. ANOVA results demonstrated the significant effect of curcumin supplementation on SOD activity in the gastric tissue of rats. After nine weeks, compared with the control group, the SOD activity of the endurance group significantly decreased (P < 0.01). In comparison with the endurance group, the SOD activity of the endurance + curcumin group significantly increased (P < 0.01). The curcumin group, compared with the control group, showed no significant changes (P > 0.05) (Figure 1).
Based on the Shapiro-Wilk test results, the normality of data distribution for serum MDA level was normal: the control (0.94), curcumin (0.82), endurance (0.36), and endurance + curcumin (0.22) groups. ANOVA results demonstrated the negative effects of curcumin supplementation on serum MDA levels. After nine weeks, compared with the control group, serum MDA level in the endurance group significantly increased (P < 0.05). Also, the serum MDA level in the endurance + curcumin group significantly decreased (P < 0.01) compared with that of the endurance group. There were no significant differences between the curcumin and control groups in terms of serum MDA levels (P > 0.05) (Figure 2).
5. Discussion
The significant decrease in gastric SOD and increase in serum MDA levels in the endurance training group compared with the control group after nine weeks of strenuous endurance training demonstrated that the high-intensity endurance training results in the destruction of the antioxidant balance in a trained body. Naturally, an increase in metabolic activity can overwhelm the endogenous antioxidant defenses and the production of free radical species may even surpass the strengthening of antioxidant defense (1). Studies showed that moderate- to high-intensity endurance exercise causes a significant free radical generation and oxidative damage to cell membranes (25). However, the benefits of exercise training to antioxidative balance seem paradoxical, considering that exercise can improve antioxidative power and increase dangerous ROS (26). Studies show that the local generation of oxygen, superoxide, and hydroxyl radicals is the prerequisite step at the beginning of the gastric mucosal injury. Reactive oxygen metabolites generated extracellularly are toxic to gastric mucosal cells (27).
The underlying etiologies of gastric symptoms include gastric emptying, acid secretion, oxidant factors, and blood supply. It seems that exercise alters these functions by influencing the regulating factors. For example, during exercise training, the muscular blood flow increases and gastric tissue receives less blood (28). It is proposed that ischemic damage is one of the causal mechanisms of gastrointestinal bleeding during and following exercise training. Also, during ischemia, xanthine oxidase enzymes may activate, causing oxidative stress by generating free radicals. In addition, the concentration of antioxidant enzymes may reduce. During reperfusion, oxidative stress also increases (10). Oxygen radicals are suggested as an important pathogenic factor that can cause the injury to gastric mucosa mainly through lipid peroxidation. Many studies demonstrated that gastric mucosal injury induced by ischemia-reperfusion are markedly reduced by the inhibition of the enzyme involved in oxygen radicals generation (27).
Since 1970, sports nutrition scientists have attempted to help athletes retain antioxidant capacity against strenuous endurance training and events. They recommend the use of natural nutrients (e.g., vitamins, minerals) instead of drugs and artificial supplements, but athletic demands exceed the ability of the body to meet these requirements with only natural nutrients (17, 29). The results of the current study showed that curcumin reduces lipid peroxidation and consequently prevents exercise-induced oxidative damage. There is probably a delicate cut-point for volume, intensity, and loading in the design of training programs for elite athletes. Based on previous researches, curcumin scavenges ROS and inhibits gastric SOD activity (19). The mechanism of the antioxidant activity of curcumin is trapping and stabilizing a variety of free radicals through the donation of hydrogen atoms (30). Comparing the control and curcumin groups, it seems that curcumin supplementation does not affect the activity of the SOD enzyme at rest. Moreover, there was no significant difference between the endurance + curcumin and control groups; it suggests that curcumin supplementation can return the SOD enzyme activity to resting level. Also, there were no significant differences in BW of rats between the control and curcumin groups as well as the endurance and endurance + curcumin groups. Significant differences between training (endurance and endurance + curcumin) and non-training (control and curcumin) groups are related to differences in activity level and caloric balance.
5.1. Conclusions
The results of the current study showed that prolonged strenuous endurance training induces oxidative stress in gastric tissue, and curcumin supplementation restores the antioxidant defense of the gastric tissue and body. Thus, curcumin supplementation is recommended for endurance athletes in order to prevent gastric inflammation and oxidative stress.