1. Background
Parkinson’s disease (PD) emerges after the destruction of dopamine (DA) secreting cells [1, 2]. The destruction of dopamine-secreting cells in midbrain disturbs other controlling centers of body movements [3]. PD is identified with four main characteristics: Resting tremor which is known as a low-range involuntary rhythmic movement, bradykinesia (dullness and movement limitation), rigidity (muscle stiffness), and postural instability [4, 5]. Disparate medicines have been offered in order for curing this disease, and among them L-dopa, which has the most usage [6], in this case, encompasses different side effects, and even the long-term consumption of L-dopa is accompanied by pharmaceutical resistance [7, 8]. Current pharmacological treatments which are preponderantly employed for symptomatic controlling, only offer short-term benefits prior to the deterioration of symptoms and pharmacological side effects [9]. As a result, prevention of this disease sounds to be a better approach for controlling and reducing the huge medical expenses [10]. PD patients have less Vitamin D level than their non-PD peers [11]. Cross-sectional studies show a correlation between vitamin D level and the intensity of PD symptoms [12]. Recent studies have demonstrated that vitamin D can be effective on muscles, immunity, endocrine glands and central nervous system (CNS) [13-16]. This steroid hormone has a significant role in the nervous system including differentiation, calcium regulation, hemostasis, neurotrophins release, brain genes activity and the metabolism of synapses enzymes [17]. It has been explored that vitamin D insufficiency contributes to pathogenesis and PD progress as well [11]. Moreover, this vitamin intervenes in the regulation of tyrosine hydroxylase (TH) gene expression and, as a result, dopamine biosynthesis [18]. The active form of vitamin D, 1, 25-dihydroxyvitamin D3 (1, 25-(OH)2 D3 ) (VD3) have physiological functions more than that of its conventional role in calcium hemostasis and bone health [19]. It has been reported that exercise exerts neuroprotection through neurogenesis and angiogenesis [20]. Meta-analyses demonstrated that physical exercise improves health-related quality, power, balance and stepping speed in PD patients [21, 22]. It is suggested that, regular physical exercise decreases the threat of neural impairments such as PD and other neurodegeneration diseases [23]. In addition, exercise accelerates the healing process of substantia nigra-striatum injury, and it also changes the dopaminergic neurotransmission in substantia nigra-striatum system [24]. Although the beneficial effects of exercise in PD treatment are still disputed, it is not cleared yet whether exercise can prevent neurodegeneration. Besides, molecular mechanisms of the possible benefits of exercise in experimental models of PD are not determined [25].It has been postulated that several trophic factors are involved in the processes of the exercise beneficial effects [26]. Neurotrophins like nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and insulin-like growth factor (IGF-1) have key roles in life, separation, correlations and neuroplasticity [3]. It has been reported that the deficiency of IGF-1 leads to the cognitive disorder and dementia in elderly [27, 28]. IGF-1 has multiple impacts on the nervous system and particularly on the structure and synaptic flexibility which is beneficial and necessary for protection and maintenance of cognitive function during senility [29]. Li and colleagues (2015) in meta-analysis of PD patients demonstrated that serum IGF-1 levels in PD patients was significantly higher than healthy control group. The authors support the hypothesis in which the increase in serum IGF-1 level can act as a biomarker for early diagnosis of PD [30]. Ebert and colleagues (2008) suggested that IGF-1 has a dual role in both increasing the hNPC survival after transplantation and trophic effects on degenerating DA neurons in rat model of PD [31]. Treatment by growth hormone (GH) increases the diameters of diencephalic (anterior-posterior, for instance) in the developing brain after birth [32]. Several studies have been conducted about VD3 supplementation and physical exercise separately on the abnormality of brain function [20, 33]. However, there are not any studies in which the prophylactic effect of VD3 supplementation and exercise on the protection of dopamine, TH and IGF-1 levels of rats’ striatum would be investigated. So, this study was designed to investigate the pretreatment effect of 4 weeks of VD3 supplementation along with forced treadmill running on the level of dopamine, TH and IGF-1 in parkinsonian rats induced by 6-OHDA.
2. Methods
In this experimental study, we provided 54 male Wistar rats (12-week old) from Pasteur institute, Iran. After transferring to the laboratory, rats, in order to adapt to the new environment, were maintained during a week in transparent polycarbonate cages at 20 - 24°C, 45% - 55% of humidity, and under a 12:12 hour light/dark cycle.
Rats were randomly divided into 8 groups (6 per group): healthy control, healthy training, Parkinson control, Parkinson training, healthy VD3, Parkinson VD3, and Parkinson VD3 training (combined Parkinson) and healthy VD3 training (combined healthy). During the research period, rats had free access to nutrition and water.
VD3 was prepared from chemical and pharmaceutical corporation KAIMAN (USA). For preparing supplement, 100 μg VD3 was dissolved in 1 mL of propylene glycol, and then it was solved in saline 9% (each mL of solution contains 1 μg of supplement) and finally, it was intraperitoneally injected every other day with the dose of 1 mL/kg BW with an insulin syringe [34].
Training groups exercised on the treadmill in a period of 4 weeks, 5 days a week and 30 minutes daily with the rate of 15 m/min, prior to injecting 6-OHDA.
Parkinson groups went through stereotaxic surgery. Destruction of striatum in rats in order for creating parkinsonian model was carried out using stereotaxic surgery injection of 6-OHDA solution into the brain’s striatum.Firstly, in order for anesthetizing the rats, ketamine and Xylazine with the ratio of 5 mL ketamine, 3 mL Xylazine were intraperitoneally injected. Then a cannula was designed in 9 mm length (7 mm plastic section and 2 mm injecting metal section) by 27 gagesyringe and the remaining 1.5 mm for 30 gage dentistry syringe which was joint to Hamilton syringe. The obtained features for creating the hole and injecting according to the paxinos atlas was as following: 2.5 mm lateral, 1 mmdownwards from bregma and 4.5 mm in depth [35]. Rats received 20 μg of 6-OHDA in0.4 CC of saline and that this solution was made from a 250 μg stock solution which was injected (0.5 μL/min) into the right striatum.
For investigating the effect of 6-OHDA injection, and confirming rats with PD, 21 days after 6-OHDA injection, rotational behavior of apomorphine induction and cylinder test were employed. Cylinder test was carried out in a cylinder with 22 cm in diameter and 26 cm height. The total number of complete rotations (360°) in opposite direction of the injured side of striatum following the intraperitoneal injection of 0.5 mg/kg of apomorphine was counted by the researcher during a period of 30 minutes recorded by a video camera. The number of injured side rotations was subtracted from opposite side which is indicative of pure rotation numbers towards opposite side. More rotation is the demonstrator of lesion intensity in terms of dopaminergic cells [36].
After performing behavioral tests, rats were anesthetized by the combination of ketamine and Xylazine, and after opening the skull of rats, striatum tissue was rapidly isolated from other parts of the brain and it was put in liquid nitrogen. After tissue homogenesis in phosphate buffered-saline (PBS) with pH 4.7, the sample was centrifuged at 10000 G for 20 minutes, and concentration levels of DA, TH, and IGF-1 of striatum were measured using commercially available ELISA assays following the instruction supplied by the manufacturer (CUSABIO Corporation kits, China).
Results were presented as means ± standard deviation (SD). Comparison between experimental and control groups were performed by one-way analysis of variance (ANOVA) which was followed by Tukey’s multiple-range post hoc test in order to investigate intergroup differences. Also, P < 0.05 was accepted as statistically significant. All statistical analysis and plotting graphics was performed using the GraphPad prism software (version 6.07).
3. Results
Evaluation of apomorphine-induced changes in rotational behavior in 21 days after injecting 6-OHDA into the striatum was performed. The number of pure rotation was 106.2 ± 15.7 turns/min in the 6-OHDA-injection group. Pretreatment with VD3 prevented the increase of rotational behavior. Furthermore, the combination of VD3 and treadmill running had protective effects against 6-OHDA injection. However, exercise group showed no significant differences compared to Parkinson group (Table 1).
aThe values were analyzed by one-way ANOVA and Tukey comparison test.
bP < 0.001 Parkinson group versus healthy control group.
cP < 0.001 Parkinson training group versus healthy training group
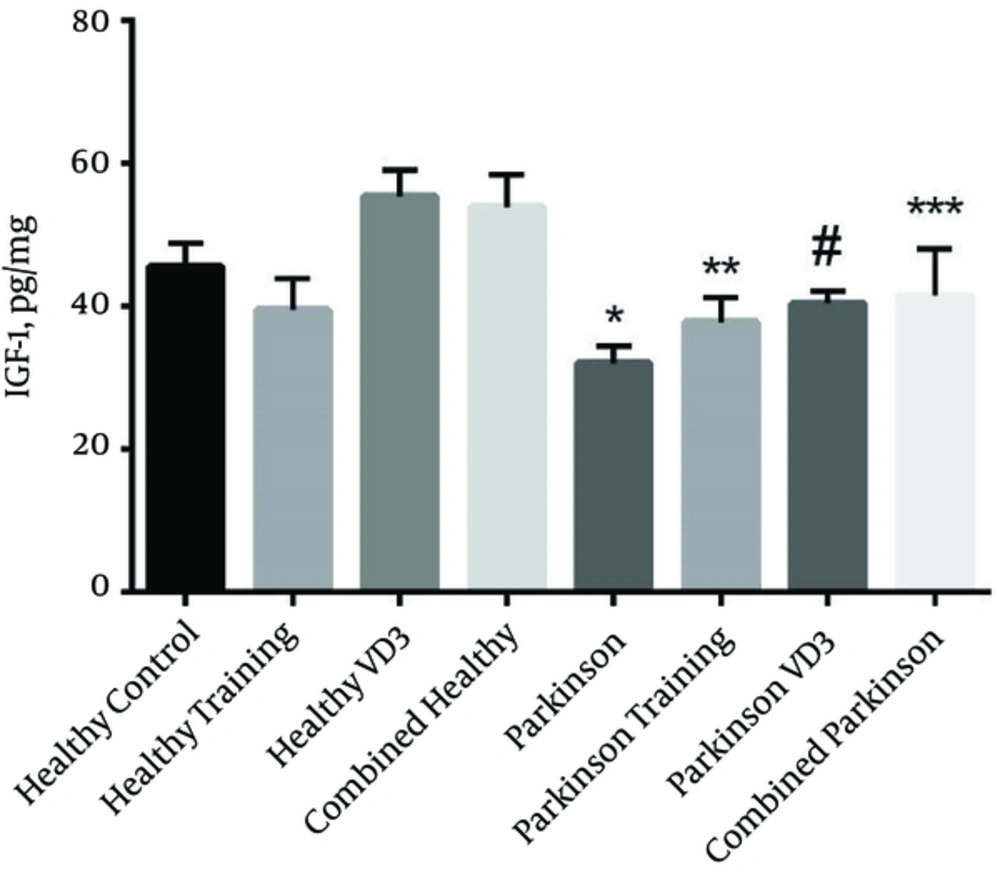
As it is depicted in Figure 1, IGF-1 level in the striatum of Parkinson rats, following the injection of 6-OHDA decreased compared to the healthy control group in which its value reached a significant level (P = 0.0002). The results obtained from one way ANOVA revealed that exercise along with supplement injection has resulted in an increase in striatum IGF-1 level of combined Parkinson group compared to the Parkinson group (P = 0.019), but the difference between combined Parkinson group and the healthy control group was not significant (P > 0.05). In addition, IGF-1 levels in combined Parkinson group was higher in comparison with Parkinson training group and Parkinson VD3 group, but its value did not reach a significant level (P > 0.05). Pretreatment injection of VD3 prevents the decrease of IGF-1 level followed by 6-OHDA injection (Parkinson VD3 group compared to Parkinson group (P = 0.038)). However, our results was indicative of no significant difference between Parkinson VD3 with healthy control, healthy training and combined Parkinson (P > 0.05). In other words, using this approach has been effective for protecting IGF-1 levels. It seems that although 6-OHDA had a noticeable impact on the IGF-1 decrease, exercise and VD3injection was able to prevent this neurotrophic factor from decreasing.
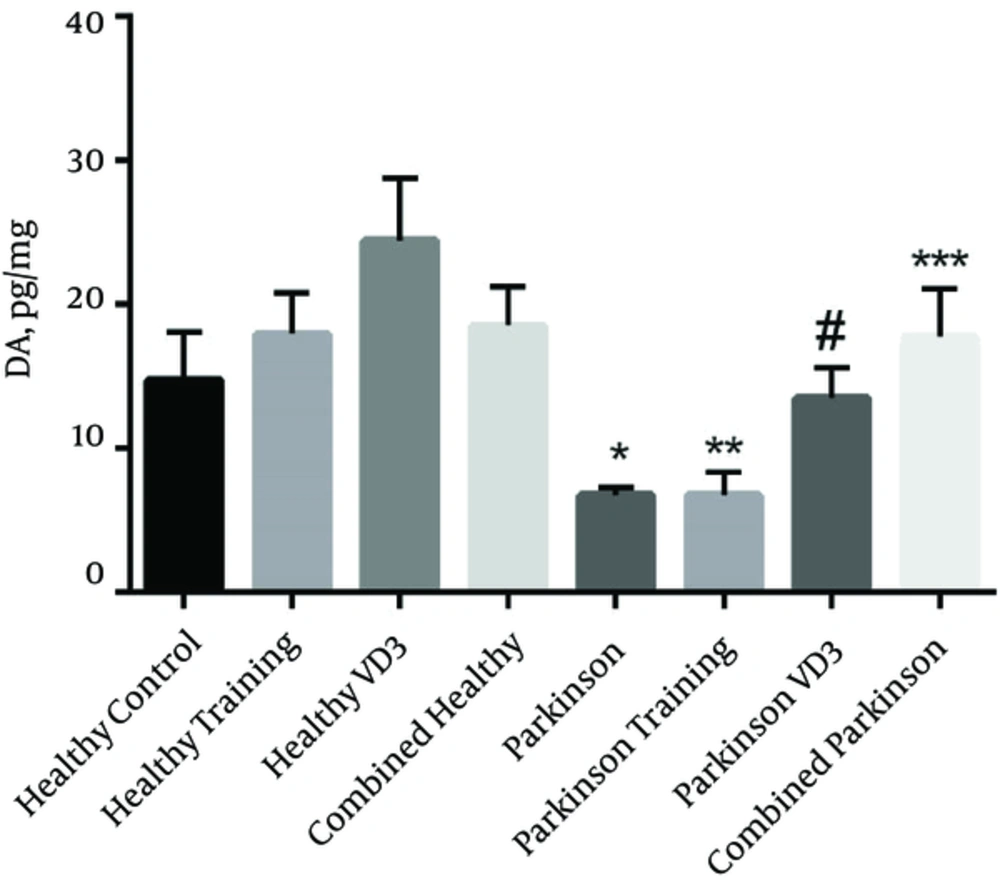
The results of one way ANOVA demonstrated that exercise in combination with VD3 have had a significant effect on DA in thestriatum of parkinsonian rats in which the values of DA in combined Parkinson group was higher than Parkinson group (P = 0.0001). But the difference between combined Parkinson group and healthy control group was not significant (P > 0.05) (Figure 2). Moreover, DA values in combined Parkinson group was higher than Parkinson training group (P = 0.0001), but it did not show a significant difference compared with the Parkinson VD3 group (P > 0.05).
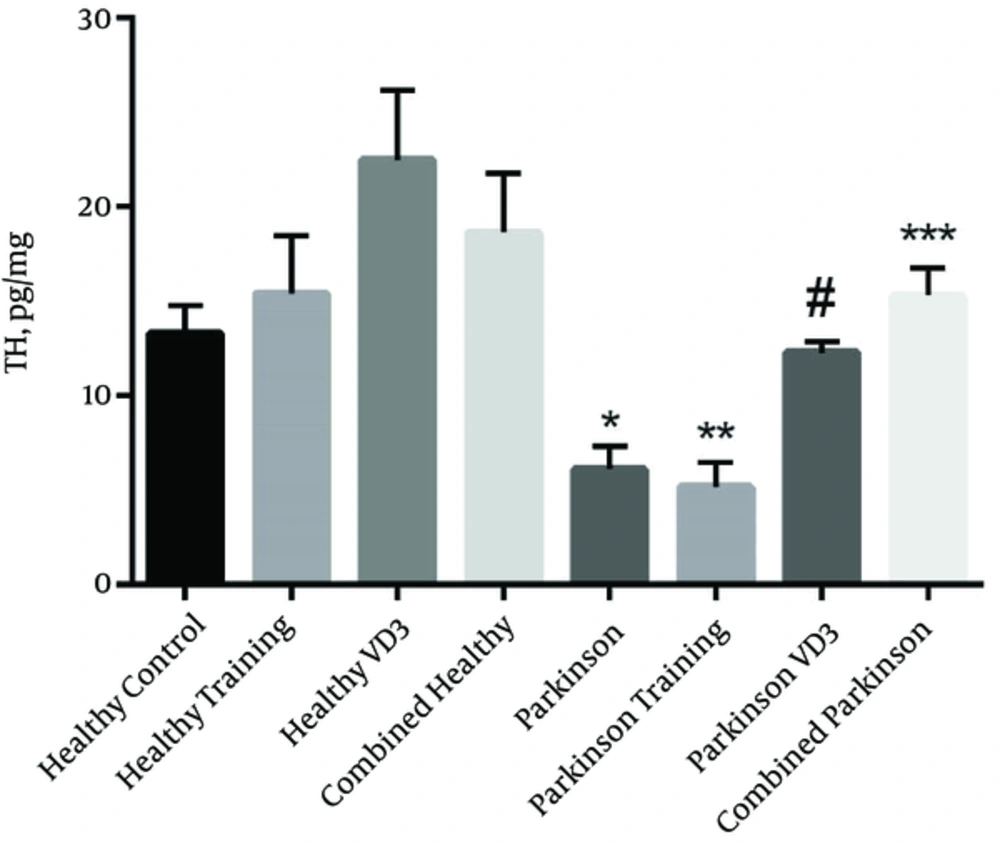
The results show that exercise with VD3 have a significant effect on TH level in striatum of parkinsonian rats in which TH levels was higher than Parkinson group (P = 0.0001). However, the difference between combined Parkinson group and healthy control group was not significant (P > 0.05). Furthermore, TH values in combined Parkinson group had a significant higher level compared to the Parkinson training group (P = 0.0001) although it did not have a significant difference compared with the Parkinson VD3 group (P > 0.05) (Figure 3).
4. Discussion
This study indicates a neural protective effect of treadmill running alongside VD3 supplementation over a period of 4 weeks. The study was conducted before receiving nervous toxin 6-OHDA on the level of IGF-1, DA and TH in the striatum of rats. It has bees postulated that their effects was more than the impacts of them separately. In fact, one of the objectives of this research was to investigate the reinforcing effect of exercise and VD3 supplementation at the same time. In other words, this question was raised whether an exercise program and VD3 supplementation together can increase the neuroprotective effect in parkinsonian rats. It is probable that owing to the impact of exercise and VD3 supplementation on IGF-1, the cooperation of these two variable results in IGF-1preservation, DA and TH resources maintenance, and consequently protection of striatum dopaminergic cells. On the other hand, possibly, simultaneous deployment of exercise and supplementation culminates in an increase in DA secretion from the remaining cells. Also, it is probable that this increase is the result of DA production in other parts of the brain and their return to striatum with backwards mechanism [37]. Offen and colleagues (2001) suggest that IGF-1 have neuroprotective effect against DA-induced toxicity, and it may have a potential role in PD treatment. This effect of IGF-1 had a correlation with increased expression of Bcl-2 [27]. Ayadi and colleagues (2016) investigated the protective effect of IGF-1 on DA neurons against oxidative stress. They find out that this protein can protect the nigrostriatal pathway in progressive PD model and this protection is preceded by activation of key pro-survival signaling cascades [38].
VD3, with regard to having active receptors in the brain, leads to the decrease of inflammations derived from neurological diseases like PD [39]. Some correlations between vitamin D levels and diseases such as cancer, arthritis, diabetes, and muscle fibers atrophy have been observed [40, 41]. In order to investigate the effect of exercise on the improvement of lost nervous dopaminergic cells in parkinsonian model rats, Choe and colleagues (2012) showed that exercise may have a protective effect on neurons against the nervous toxin-derived death. The results of mentioned study demonstrated that exercise deactivates glycogen synthase kinase 3β (GSK3ß) through phosphorylation that can be responsible for protective effect of exercise on 6-OHDA-derived cell death [4]. 6-OHDA exerted its neural destructive effects by increasing oxidative stress. Regarding the presented articles, it seems that the results acquired from combining exercise and VD3 supplementation are due to the anti-inflammatory effect of these two independent variables. It is probable that the anti-inflammatory effect of exercise and VD3 is in the form of both indirect (neurotrophic factors mediation), and direct (increase in dopamine production by dopaminergic cells of other parts of the brain and undamaged part of the striatum). Although it has been demonstrated that exercise alone can prevent the inflammation [42-44], these circumstances decrease by increasing intensity and exercise-derived stress, and it causes more tissue injuries including the brain [45]. VD3 can stimulate the release of reactive oxygen species (ROS) inhibitory factors such as lipoxygenase [33, 46-48]. Furthermore, VD3 can lead to thereduction of ROS in the liver of rats through its capability in reducing lipid peroxidation [49]. It seems that the anti-inflammatory effects of combining these 2 factors may reduce the nervous and muscle injury. Probably, combining exercise and VD3 supplementation have indirect and direct effects sothat VD3 causes the exercise and 6-OHDA-derived injuries to decrease. On the other hand, exercise can indirectly increase the production of neurotrophic factors, and prevents this injury [26, 50, 51]. The other possibility that can be mentioned for the combined effect is referred to the exercise-derived hormone response. It has been revealed that an increase in parathyroid hormone is secreted by parathyroid glands in response to the VD3 reduction in order for increasing intestine absorption and activating it. So it seems that exercise can increase parathyroid hormone [52, 53]. This exercise-derived increase can be occurred in order to compensate for VD3 reduction. In other words, it appears that exercise leads to the attenuation of mentioned physiological functions in the brain by its negative impact on VD3 level. Possibly, VD3 supplementation and exercise can separately prevent the exercise-derived reduction of VD3, and so do that for physiological functions reduction of VD3 in the brain.
The result of this research showed that the injection of VD3 culminates in an increase in the resistance of striatum DA cells against the oxidation-derived damage created by 6-OHDA. On the other hand, exercise and VD3 supplementation can increase the level of DA, TH and IGF-1 in the striatum of parkinsonian rats. However, exercise alone did not have significant effects on the levels of these variables, and also on the cylinder rotation test results.