1. Introduction
Chylothorax is defined by the leakage and accumulation of lymph into the pleural cavity. This complication has several causes and be classified as neoplastic, congenital, traumatic, idiopathic, spontaneous or in post-thoracic surgery. Any procedures within the thoracic cage that associate with damage to thoracic duct or its tributes may be associated with chylous pleural or pericardial effusion. The risk factors predicting for post CABG surgery includes left internal mammary artery (LIMA) harvesting central venous pressure (CVP) line insertion, snaring of superior vena cava (SVC), ascending aorta releasing from neighboring organs, increasing SVC pressure, postoperative thrombosis of SVC, increasing left a trial pressure, and combined atrial septal defect with pulmonary hypertension [1-3]. The careful literature, searching revealed that incidence of chyothorax following intrathoracic surgery is in the range of 0.25 to 0.50% [4, 5]. It is known that, its occurrence after CABG is extremely rare, and no case of chylothorax following OPCAB has been reported so far. We have not found any reported case of early chylous effusion after an OPCAB in patient with preoperative hypertriglyceridemia in medical literature. Therefore, we describe this extremely rare case that has some unique presentation such as occurrences after OPCAB, occurrence within three hours of an OPCAB, association with treated hypertriglyceridemia and spontaneous regression of effusion in 10th postoperative day. We exhibit not only the anatomical and physiological basis for its occurrence, but also we suggest a new risk factor (hypertrigyceridemia) for developing of chylothorax in an old procedure (OPCAB) that renewed in recent decade.
2. Case Presentation
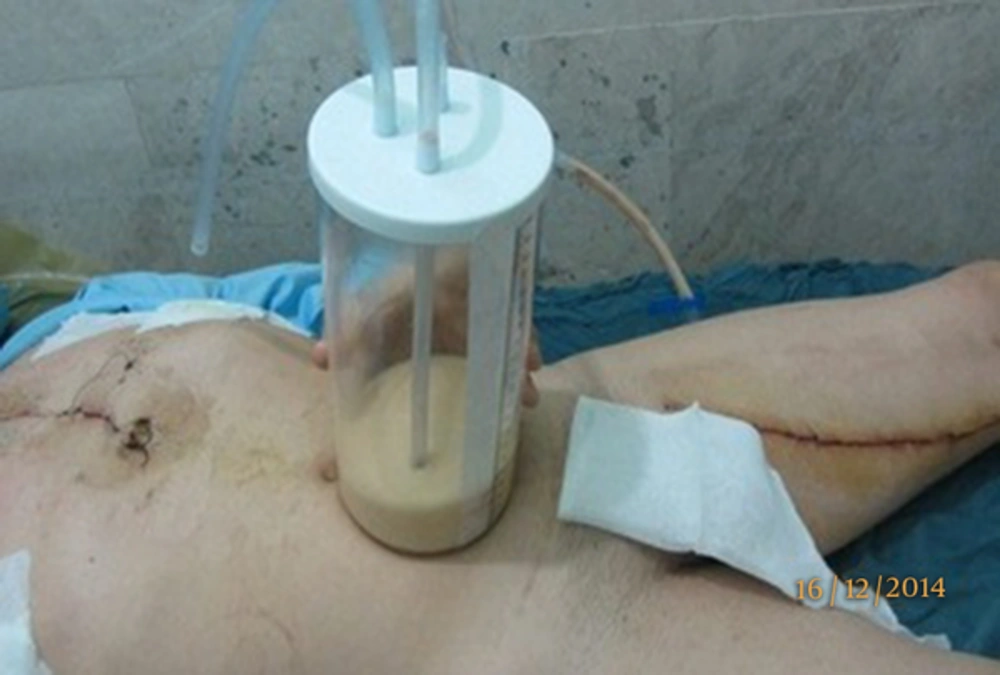
A 43-year-old male, with coronary artery disease (CAD), underwent angiography that showed stenosis in the left anterior descending (LAD), left marginal arteries (LCX) and right coronary artery (RCA). The patient scheduled for OPCAB and surgery performed by conventional median sternotomy. A segment of saphenous vein graft was anastomosed to the LCX and RCA sequentially and the internal thoracic artery (LIMA) was grafted to the LAD. Within three hours of ICU admission, the milky effusion (in opposed to usual blood stained effusion) was observed in left hemithorax chest bottle (500 mL in the first three day of operation, 400 mL in the 4th to 7th day of operation, 250 mL in the 8th day and 100 mL in the 9th day and 30 mL in the 10th of operation). A chest X-ray, showed pleural effusion (Figure 1). The patient was placed on a no-fat diet on the first day of operation. A puncture was made in the chest tube containing milky effusion and biochemical exam of draining fat droplets was exhibited in the Table 1. With conservative management, i.e. low fat diet and continuation of anti-hyper triglyceridemia drug, the early recovery was uneventful and milky effusion was ceased in the 10th post-operative day and the patient was discharged on 13th day of surgery. On 3th month of follow-up, no recurrence of effusion on chest X-ray control was found. This case report has three unique features 1-occurrence after OPCAB, 2- association with treated hypertriglyceridemia, 3-early occurrences after 3 hours of operation.
| Variables | Chest Tube Fluid | Serum |
|---|---|---|
| Leukocyte count, cells/mm3 | 4500 | 7600 |
| Neutrophils, % | 0 | 70 |
| Lymphocytes, % | 96 | 30 |
| Monocytes, % | 3 | 4 |
| Glucose level, mg/dL | 150 | 102 |
| Total protein level, g/dL | 2.6 | 5.7 |
| Cholesterol level, mg/dL | 50 | 167 |
| Triglyceride level, mg/dL | 2400 | 500 |
3. Discussion
There are many etiology, such as LIMA, RIMA harvesting, thymus handing, aortic arch surgery, snaring of SVC or IVC, mitral stenos, ASD surgery, pulmonary hypertension and others rare causes, that may be followed by chylothorax in cardiac surgery [4, 5]. Incidence of chylothorax after CABG is a few and in addition, our case is the only one of twenty six of other cases of chylothorax following CABG that have been found in the medical literature. None of them occurred after OPCAB. Another unique feature of this complication is time of occurrence within 3 hours that it’s very surprising as first few hours drainage will be blood or blood stained. Usually suspicion arises after 24 hours observing milky copious drainage. It’s very unusual finding. If we consider huge number of CABG that performed in the cardiac surgery centers in the world, reported cases of chylothorax is an exceptional complication. The rarity of this complication in OPCAB could be explained by lower chance of injury to the thoracic duct in this type of surgery. In opposed to OPCAB surgery some additional procedures may be performed in on-pump such as, snaring of SVC, handling of ascending aorta, aortic cross clamping that may be associated with thoracic duct injury [6]. Other possible etiologies have been proposed, such as increased superior vena cava pressure due to use of tapes or to venous thrombosis during cardiopulmonary bypass. Location of the thoracic duct in the superior mediastinum close to SVC, ascending aorta, aortic arch and to the left subclavian artery predispose it to a traumatic injury during CABG. In addition, injury to large branch of thoracic duct may be occurred during dissection of thymus or LIMA harvesting close to subclaveian artery. Another procedure that may be associated with injury to thoracic duct is insertion of central venous pressure line from left internal jugular vein. In the neck, thoracic duct has an upward curved course and after down ward rotation, posterior to the first portion of the left subclavian artery, it jointed to the left internal jugular vein that may be injured by repeated trauma of CVP line insertion [7]. During OPCAB, lymphatic ducts may be injured in the region of the thymus or near the origin of the internal thoracic artery, which is destination site of thoracic duct during harvesting of LIMA. In other hand LIMA has a rich collateral network of lymphatic ducts that originated from inter costal lymphatic chain. These collateral chains due to congenital abnormality may become more prominent in some patients. In addition, abnormal course of thoracic duct has been documented in more than fifty percent of patients. Some patients have two thoracic duct with mirror images map that may end at theirs course to the hemi azygus, azygous, inominate, intercostal veins, and the subclavian-jugular venous junction .The most important procedure during OPCAB that may lead to thoracic duct injury is LIMA harvesting [8]. lymphatic collateral and its tributaries not only have close proximity to destination site of thoracic duct in the origin of LIMA but also receive many collaterals lymphatic chain from inter costal network that may injured during harvesting. Another risk factor of chylothorax during harvesting of LIMA is electro cauterization. In opposed to blood vessels that electrocautery by coagulation of blood protein led to hemostasis, in lymphatic with low content of protein, electrocautery burns intra lymphatic lipids and blow up them and ending in lymph leakage. In opposed to others case reports that Chylothorax usually started to drainage some days after surgery when oral intake begins or fatty meal served, in our case the initial symptoms was appeared within three hours of surgery. This sudden beginning of chylous effusion in ours patient explained by severe hyperlipidemia. Despite preoperative management of hyperlpidemia by related drugs, the patients fasting serum triglyceride level was 250 mg/dL. Hyperlipidemia has a role of fatty meal that causes early confession of lymph leak in postoperative periods. The most common sign of chylothorax is blunting of costo-phernic angle or mediastinal widening. Mild case of chylothorax has not brilliant sign and symptom, however in moderate to server cases, dyspnea, orthopnea, fever, weight and appétit loss occurred. Laboratory exam of milky fluid revealed high content of fat that may associates with presence of pancreatic lipase, amylase and deoxyribonuclease. Peripheral smear of lymph also shows free fat in microscopic exam. Lymphocytes are the main cellular component of lymph by in the range of 5,000 per mm2. The Red blood cells content will be low and theirs range could be between 20 - 50 cells per mm2. The initial treatment of chylothorax is conservative approach, to minimize chyle formation, to prevent the immune deficiency, and to maintain adequate drainage as well as to replace a high-fat diet with high content protein diet which is absorbed directly in the portal system without passing through the thoracic duct [9-12]. If the drainage remains high in spite of therapy, total parenteral nutrition must be indicated. Surgical intervention will be considered only if there is incomplete drainage or continuous loss of chyle. The early surgical treatment of voluminous leakage is indicated when adhesion is not widespread but when surgical approach was delayed a plentiful fibrinous adhesion prevent easy finding of leakage site. In this case, pleural cleaning and pleurodesis is a good choice. In an anatomical study, Riquet et al. reported that LIMA dissection close to the left anterior mediastinal lymph node chain caused injury to these chain and leakage to thorax [13]. Like to peripheral vein, lymph vessels have valve and lymph back-flow is impossible during proximal injury to these lymph chains. Congenital lymph valve insufficiency, another cause of post-surgical chylothorax is very rare phenomenon and was not reported in post CABG so far. Another cause of thoracic duct injury is surgeon try to maximize the conduit's length near the proximal end of the LIMA pedicle [14, 15]. In conclusion, preoperative hyper triglyceridemia treatment is an important tools for avoiding, chylothorax occurrences in post OPCAB period. However as a rare complication after OPCAB, like our case, where the early diagnosis was made, hyperlipidemia treatment by drugs is extremely useful, because it is an efficient and less invasive method which allows for rapid reducing of chyle volume. A careful diet is essential for a good clinical outcome.