1. Background
Cystic echinococcosis (CE) is an ancient, still relevant zoonotic infection worldwide, rendered by the so-called hydatid cysts, as larval stages of Echinococcus granulosus (E. granulosus) (1). The interaction between canid and herbivore species is the cornerstone of parasite maintenance in different ecological niches (2, 3), which significantly impacts socio-economic consequences in endemic territories (4). Humans, however, are considered dead-end hosts for hydatid cyst, which are accidentally affected by egg ingestion; particularly, in rural areas where there is close contact with dogs and weak hygiene practices (5). Owing to traditional animal husbandry in various regions of Iran and the accessibility of canids to abattoir wastes, Iran is an endemic region for CE, like other Mediterranean littoral territories (4, 6). It has been estimated that the financial burden of CE in Iran is US$ 232.3 million annually (7).
Liver and lung are the most parasitized organs in the human body; however, the clinical picture of the infection is significantly variable on the basis of affected site, cyst condition, and size (8). Echinococcal cysts with a slow growth rate are often well-tolerated and asymptomatic until they cause organ dysfunction due to enlargement (4). Furthermore, the sudden cyst rupture would release a large volume of antigenic molecules, triggering antigenic reactions such as fatal anaphylaxis (9). Notwithstanding, a history of sheep-dog exposure in a CE endemic zone for a patient having a cyst-like mass is of medical importance for diagnosis, the hydatid cyst should be differentiated from other cyst-like structures, including abscesses and neoplasms. For this aim, combined utilization of non-invasive immunodiagnostic tests and imaging approaches such as ultrasonography are recommended (4).
Despite the fact that unprecedented molecular tools are more preferred to detect CE in suspected patients (10), immunodiagnostics are still considered, especially in underdeveloped countries and for determining infection status, mass screening surveys, and treatment follow-up (11). Patients infected with hydatid cysts develop measurable humoral and cellular immune responses, including a high level of IgG class followed by trace IgM, IgA, and IgE classes in some individuals (11). Antigens 5 and B are two predominant molecules of hydatid cyst fluid, which are widely used for CE serodiagnosis using various serologic tests, including immunoelectrophoresis and enzyme-linked immunosorbent assay (ELISA) (12).
2. Objectives
Owing to different sensitivities and specificities of Commercial ELISA kits, we developed an experiment to assess the efficacy of native antigen B (Ag B) in comparison to a commercial ELISA kit in the diagnosis of hydatidosis-specific IgG among surgically-confirmed cases, heterologous, and control groups in Khuzestan province, Southwestern Iran.
3. Methods
3.1. Subjects and Serum Collection
The current comparative investigation was designed on the basis of 90 serum specimens, of which 50 sera were obtained from surgically approved CE patients during the last 5 years, 20 heterologous serum samples from individuals having anti-Toxoplasma antibody, giardiasis, hepatitis, kala-azar, icteric, AIDS, cancer patients as well as 20 sera from healthy individuals approved by the results of immunological tests, hematology, biochemistry, hormone, and liver. Specimens were kept at -20°C for further serological analysis.
3.2. Native Ag B Preparation
Hydatid cysts isolated from slaughtered sheep were washed with distilled water then aspirated. Only cysts with clear liquid were included and those having purulent and/or turbid contents were excluded. The purification of Ag B was performed based on Oriol et al. study (13). In brief, 100 mL of centrifuged (2000 rpm, 10 minutes) hydatid cyst contents was dialyzed against acetate buffer (5 mM, pH = 8, and 4°C). The dialyzed solution was centrifuged at 5000×g for 30 minutes (4°C) and the solubilized protein concentration was determined using Bio-Rad assay.
3.3. Checkerboard and in-House ELISA
Checkerboard using indirect ELISA method was used to detect the optimum concentration and dilution of antigen and serum as the following: (1) antigen serial dilution in carbonate/bicarbonate buffer (pH = 9.6) was coated, (2) Triplicate micro plate washing by 3% phosphate buffered saline (PBS)-Tween20, (3) Blocking procedure was done by adding 200 µL of 3% skimmed milk in PBS and one hour incubation at room temperature (RT), (4) The plate was washed and positive and negative control sera in PBS were added as serial dilution, (5) Washing was repeated and 100 µL antihuman globulin alkaline phosphatase (1:2000) was added to each well, followed by one hour incubation at RT, (6) Again washing, then 100 µL P-nitrophenil phosphate substrate was added to wells and the plate was incubated in a dark place for 30 minutes (14).
Following the evaluation of the appropriate dilutions of serum and antigen, titration and optimum dilution of the conjugate were assessed. For this aim, various dilutions of conjugate from 1:400 to 1:32000 were reacted to serum (1:100) and antigen (5 µg/mL). Also, two time intervals, including 20 minutes and 30 minutes were examined to estimate the best incubation time following the substrate addition, then optical density was read by Stat Fax® 2100 Microplate Reader (Awareness Technologies, USA) (14).
3.4. Commercial ELISA Test
In this experiment, we employed Vircell (Granada, Spain) indirect immunoenzyme assay to be compared with our set up ELISA. At first, both positive and negative cut-off were added to 96-well plate. Diluted (1:50) serum samples were added to the respective wells in duplicate. After 2 minutes shaking, the plate was covered and incubated at 37°C for 45 minutes. Plate contents were depleted and washing solution was added three-times for washing procedure. Following the addition of 100 µL anti-human IgG alkaline phosphatase conjugate, a 30-minutes incubation was done at 37°C. The plate was washed, then 100 µL substrate was added to each well and incubated at RT in a dark place for 20 minutes. Ultimately, 50 µL stop solution was added to each well and the absorbance was determined by Stat Fax® 2100 Microplate Reader (Awareness Technologies, USA) in 450 - 620 nm.
3.5. Cut-Off Point Determination
To infer positive and negative results for hydatid-specific IgG cut-off points were used. The cut-off index for commercial ELISA test was as follows: < 9 as negative; 9 - 11 as doubtful; and > 11 as positive. The cut-off point for in-house ELISA experiment was defined as a sum of two-fold standard deviation and the mean optical density of all negative samples.
3.6. Statistical Analysis
In order to properly report the obtained results, sensitivity and specificity were used. The kappa statistical test was employed to compare qualitative characteristics of both tests. A P value > 0.05 was defined as statistically significant. All analyses were performed using SPSS statistical software (version 22).
Positive likelihood ratios = (1-specificity)/sensitivity
Negative likelihood ratios = (1-sensitivity)/specificity
3.7. Ethical Issues
All of the participants in this study were diagnosed to be qualified for hydatid cyst operative surgery and received no medical interventions. The information retrieved from each patient was confidential and merely delivered to the patient himself/herself. The current investigation was conducted using blood samples obtained for routine laboratory tests and no further blood samples were taken.
4. Results
In this study, a total of 90 sera comprising case (CE-infected patients), heterologous (infected to non-CE diseases) and control (healthy individuals) groups were collected and evaluated regarding hydatidosis-specific IgG by native and commercial ELISA tests.
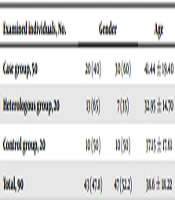
Age distribution of examined fellows was enclosing: 12 individuals (13.33%) under 20; 43 individuals (47.78%) 21 - 40; 22 individuals (24.45%) 41 - 60; and 13 individuals (14.44%) over 60. Table 1 demonstrates the distribution of examined individuals in accordance with age and gender. Moreover, operation revealed the most parasitized organs by hydatid cyst in some patients, as follows: liver (16 cases), lungs (10 cases), liver and lung (6 cases), kidneys (4 cases), leg bone (1 case), liver and kidney (1 case), liver and hip (1 case), kidney and lung (1 case). Checkerboard this experiment determined optimum dilutions for antigen and serum as 1:400 and 1:100, respectively. Also, 1:2000 conjugate dilution yielded the optimum absorbance of positive and negative controls, conferring it as the best dilution for conjugate. Moreover, 20 minutes time interval was more optimum than 30 minutes for incubation after adding the substrate.
| Examined Individuals, No. | Gender | Age | ||
|---|---|---|---|---|
| Male | Female | |||
| Case group | 50 | 20 (40) | 30 (60) | 41.44 ± 19.40 |
| Heterologous group | 20 | 13 (65) | 7 (35) | 32.95 ± 14.70 |
| Control group | 20 | 10 (50) | 10 (50) | 37.15 ± 17.61 |
| Total | 90 | 43 (47.8) | 47 (52.2) | 38.6 ± 18.22 |
aValues are expressed as mean ± SD or No. (%).
Using commercial ELISA kit and native ELISA test, 22 out of 90 and 52 out of 90 sera were positive for hydatid-specific IgG, respectively. A bi-conditional state was considered for determination of sensitivity and specificity. The first aim was to define the sensitivity on the basis of cases with previous surgery.
In the case group, all 50 patients with surgically approved CE were positive with in-house Ag B ELISA test, whereas merely 22 cases were positive using commercial hydatidosis ELISA assay (Table 2). Since fellows in the control and heterologous groups were not inspected by operation for hydatid cyst infection, we could not characterize the specificity. In the second state, the commercial kit was used as the gold standard and sensitivity and specificity were 97% and 96%, respectively, according to the manufacturer’s instructions. Given that all 22 positive samples in commercial test were also positively detected by our in-house ELISA kit, both assays possessed the same sensitivity (97%). In the control group, among 20 negative samples reported by the commercial kit, 19 cases were considered negative using in-house test, thereby yielding a specificity of 95%. In the heterologous group, 19 specimens were negatively reported for hydatid-specific IgG by in-house assay, while 20 samples were detected as negative by the commercial test, asserting a 95% specificity.
| ELISA Test | Compared to Operation, % | Compared To Commercial Kit, % | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Commercial | 44 | - | 97 | 96 |
| In-house | 100 | - | 97 | 95 |
Table 3 describes the IgG status in sera of the case, heterologous, and control groups. One sample from the control group and one specimen from the heterologous group were positive by our in-house ELISA, while the whole samples of both groups were negative by commercial ELISA assay.
| Examined Groups | No. of Samples | IgG Positive by Commercial ELISA | IgG Positive by in-House ELISA |
|---|---|---|---|
| Case | 50 | 22 (44) | 50 (100) |
| Control | 20 | - | 1 (5) |
| Heterologous | |||
| Individuals with anti-Toxoplasma antibodies | 1 | - | - |
| Giardiasis | 5 | - | 1 (20) |
| Cancer | 4 | - | - |
| Hyper bilirubinemia | 1 | - | - |
| Kala-azar | 4 | - | - |
| HIV and Hepatitis | 3 | - | - |
| Hepatitis | 2 | - | - |
aValues are expressed as No. (%).
5. Discussion
From centuries ago, human hydatidosis is considered a prevalent parasitic zoonosis with common asymptomatic characteristics of the early infection and/or long after CE establishment, which renders difficult diagnosis (1, 11); however, its identification and management have been considerably improved during last decades by the advent of sophisticated laboratory diagnostics, including serological and imaging techniques, which are primary detection methods (4). Notwithstanding, the cystic stage of E. granulosus handles various mechanisms, consisting of antigenic variation, antigenic mimicry, immunologic diversion, immunologic subversion, and immune indifference for escaping from the host defense (15), a level of immune response of Th2 type and IgG1, IgG4, and IgE isotypes is usually elicited by hydatid cysts (11, 16) that is principally dependent on the host species, immune status, infected organ, and the involved genotypes and/or haplotypes of the parasite (16). The accurate recognition of such responses are substantially critical for parasite clearance as well as developing immunodiagnostic kits and efficacious vaccines (11, 16). Serodiagnosis of CE using a potent antigenic source i.e. hydatid cyst fluid provides early chemotherapy, more effective treatment, and post-operation follow-up (12). For this aim, multiple serologic tests, including Casoni, latex agglutination, indirect hemagglutination, complement fixation, enzyme immunoassay, immunoelectrophoresis, western blot, and ELISA assays have been developed in order to accurately detect the hydatid cyst-specific antibodies or antigens (17, 18). Owing to the possible cross-reactions with other relatively-closed helminth parasites such as E. multilocularis, Taenia hydatigena, and Taenia ovis, the quality and efficacy of CE serodiagnosis in suspected cases are still controversial (12). Herein, we have evaluated the comparative sensitivity and specificity of a designed in-house ELISA using native Ag B and a commercial ELISA kit to detect anti-hydatidosis IgG among surgically-confirmed cases, heterologous and control groups in Ahvaz, southwest of Iran.
Altogether, sera rom 50 hydatid cyst patients, 20 healthy individuals and 20 fellows with non-CE diseases were employed in our investigation. Considering surgical intervention as the gold standard, the sensitivity of native Ag B ELISA and the commercial kit was 100% and 44%, respectively. Among other examined fellows, a subject out of the heterologous group infected with giardiasis and one out of the control group were reported to be positive with native assay; as a consequence of the highest sensitivity of this test, both cases should be monitored using imaging techniques to confirm or reject the contingency of hydatidosis. Our findings on the sensitivity of native ELISA was consistent and higher than similar studies having a range of 40 - 81 patients with hydatid cyst (the case group) and 85-89% sensitivity (19-21). Some investigations documented a sensitivity range of 84% to 96%, especially because of type of antigen, antigenic source, and purification method (20, 22, 23).
Despite the commercial kit is the gold standard, all heterologous and control individuals were negative by such kit; however, a healthy person and a heterologous individual with giardiasis were positive using native Ag B ELISA test. Accordingly, sensitivity and specificity for IgG assessment by in-house test was 97% and 95%, respectively. Other investigations also reported 95-98% specificity rates (19, 24). Cross-reactions are of utmost importance in specificity appraisal of a particular assay. For hydatid cyst immunodiagnostics developments, sera from cysticercosis, schistosomiasis, onchocerciasis and sarcoidosis patients are frequently used for cross-reaction assessments. Thereby, lack of such infections in the area and/or inaccessibility to sera of such patients would entail the higher specificity of designed in-house assays.
In the current study, most infected cases were between the ages of 21 and 40 that is consistent with findings of similar studies. Haniloo et al. documented the most cases of infection in the age range of 10 and 40, while hydatid cyst was less detected among over 50 individuals (25). Furthermore, Aflaki and colleagues discovered most hydatidosis patients between the ages of 20 - 30 and 30 - 40 (26). On the one hand, these age ranges are probably most exposed to infection sources; on the other hand, the chronic nature of CE and its long-lasting incubation period make it more prominent in such ages. Nevertheless, CE establishment likely occurs in the childhood, as the seroprevalence of hydatidosis in the first two decades of lifetime is remarkable analogous to middle Ages. Children and younger adults in endemic regions are readily infected with hydatidosis, due to the low hygiene practices and contact to infective canids (27, 28). Additionally, hydatid cyst seroprevalence noticeably is decreased in individuals with over 50 ages, which possibly occurs owing to the lower exposure to sources of infective eggs, cyst inactivation and/or self-cure. The current investigation showed a 3:2 ratio for female and male fellows, respectively, suggesting more contact of females to egg-shedding dogs or egg-contaminated vegetables. In Aflaki et al. study, also, females were 1.5 fold more infected to echinococcal cysts than in females because of close contact to dogs (26). However, Rafiei and Craig study using ELISA on 4,569 individuals found no statistically significant difference between two genders regarding hydatidosis (29).
5.1. Conclusions
Our native in-house ELISA method using native Ag B showed 100% sensitivity among surgically-confirmed hydatidosis-affected patients as well as the heterologous and control individuals. Consequently, if two hydatid-specific IgG-positive fellows in the control and heterologous groups are actually infected with the cystic stages, the specificity of such antigen will be very considerable and the test is a reliable assay. Further studies must be relied on IgG1 and IgG4 detection besides total IgG to better validate the laboratory diagnosis of hydatid cyst in suspected cases. Moreover, initial screening by imaging methods prior to Ag B serodiagnosis in epidemiological investigations could be an alternative to surgical-related hydatid cyst records.