1. Background
Healthy babies experience pain during procedures such as blood sampling for metabolic screening, vitamin K injections or hepatitis B vaccination. In addition, the sick or preterm infants undergo further repetitive painful procedures that are essential to their lives (1). According to the CDC 2010 Handbook, children in the first six years of life should have 30 or more injections, most of which occur in the first 6 months of life (2).
It was previously thought that babies were unable to understand pain due to the lack of central nervous system development (3-6). Nowadays it has been shown that physiological, anatomical and chemical-related neuropathic structures have been completed even several weeks before birth (4); and infants have lower levels of endorphins in comparison with children and adults and their natural system of pain control is limited (7).
There are ways to manage the pain of surgery, medical illness and major procedures, ways of preventing or reducing pain from minor medical procedures (e.g. heel lance and venipuncture) have, until relatively recently, been lacking (8). Although the pain caused by vaccination is short, studies have shown that this short pain causes discomfort to the baby, parents and vaccinator (9) and in addition to the immediate effect it has short-term and long-term effects (10-14). Short-term effects include decreased oxygenation hemodynamic instability and increased intracranial pressure (12). Though the long-term effects of pain and stress are not well known, some studies have shown that uncontrolled stress and pain in neonates can lead to some changes in the central nervous system (15), these changes include permanent damage to cognitive development of learning, memory (13), IQ intelligence (10) and behavior. Also these pains can lead to increased physical harm (13) anxiety, emotional complications, agility and child-related problems (12).
Prevention or reduction of pain is one of the vital goals in infant medical science. As far as possible avoiding unnecessary painful procedures, and when an aggressive procedure is necessary, it is important to control the pain by pharmacological or non-pharmacological methods (1).
Many researches have been conducted today on non-pharmacological pain relief in infants including the use of sucrose. In 2012, Carrie and colleagues conducted a study to assess the effect of 50% and 75% oral sucrose on vaccine-induced pain in 2, 4, and 6 month-old children. The results of this study showed that there was no significant difference between age groups in terms of pain score and crying time (16).
The study of Moradi and colleagues in Iran aimed at comparing concentrations of 20 and 50% sucrose to the pain caused by hepatitis B vaccine showed that Sucrose 50% significantly decreased pain at 2 and 7 minutes after administration, but the results of comparing different concentrations showed that the effect of sucrose relief was not increased with increasing concentration (17).
Another study was conducted to assess the effect of 20% sucrose on the pain caused by the injection of hepatitis B vaccine. The results showed that NIPS scores were not significantly different between two groups in case and control groups 2 minutes after administration of sucrose; but 7 minutes after the administration of sucrose the intensity of pain in the case group was significantly decreased (18).
The problem that has been observed in previous studies is the use of various amounts and concentrations of sucrose on different procedures which has limited efforts to stabilize sucrose concentration to relieve infants’ pain. And according to previous researchers, no study has been performed on the final concentration of sucrose (1, 16). On the other hand, some researchers believe that the effect of sucrose increases with concentration which requires more studies (19). It can be proved by the fact that sucrose can also reduce the pain caused by injections in infants’ wards and many complications caused by pain in newborns.
2. Objectives
The aim of this study was to determine the effect of sucrose 30% and 50% on the pain induced by intramuscular injection of hepatitis B vaccine and the effect of different concentrations of sucrose.
3. Methods
This is a triple-blind clinical trial, that was carried out after obtaining permission from the Ethics Committee and the necessary permissions from the university’s Vice Chancellor of Research and Head for the Department of Obstetrics and Gynecology on healthy term infants born in Amir al-Momenin Hospital in Semnan.
This study was registered at the Center for Clinical Trials (N1 138902223920). The criteria for entering the study were: birth at 37 - 42 weeks of pregnancy, weight over 2.5 kg, age 1 - 2 days, Apgar score (first minute) 7 - 10 and no previous painful intervention such as cardiopulmonary resuscitation, blood sampling, circumcision and injection. Exit criteria included: feeding the baby 30 minutes before injection, taking acetaminophen on the same day and naloxone or phenobarbital in the last 48 hours, taking any narcotic substance by the mother, having cardiorespiratory, evolutionary, neurological problems, , and infants who have been intolerant to fructose or sucrose.
This study was carried out in the vaccination unit of the obstetrics and gynecology department on infants who were vaccinated at birth. The number of samples based on previous studies, considering 99% confidence level, power of 95% and the formula for comparison of the mean, 91 patients were estimated who were randomly divided into two groups of 30 cases and one control group (31 neonates). After receiving a written consent from one of the parents, the baby was transferred to the vaccination room and the baby covers were removed before the start of the examination and behavioral responses using the Neonatal Infant Pain Scale (NIPS) was determined by direct observation of the infant by a nurse associate who had a history of work in the neonatal department and was trained by the researcher on how to use the scale (all observations were done by one person).
Then 2 mL of solution 1 (30% Sucrose) or 2 (50% sterilized sucrose) or 3 (sterile water) using a 2 cc syringe without a needle and while the head was slightly raised from the corner of the baby’s mouth the anterior mouth was demonstrated and two minutes after administering sucrose to all infants in each of the 3 groups of 0.5 mL of hepatitis B vaccine, with Insulin syringe was injected into the vastus lateralis muscle (in the middle third). All injections were performed by a qualified nurse. Then immediately after the needle was removed and 5 minutes after injection the behavioral response was measured for the second and third times by the same investigator. The random allocation of newborns to different groups was as follows: that According to the entry of the infants into the vaccination unit was given to the first infant of solution number one to the second infant of solution number 2 and to the third baby solution number 3. Sucrose and sterile water were prepared in completely identical containers and the investigator, co-author prescribing sucrose and the Statistics Consultant did not know the solution type.
Parents were not allowed to enter the vaccination room with written consent and prevented from hugging, kindnessing and calling the infant during the investigation. If the parents refused to participate in the study only vaccine infants were injected and taken out of the sample. All people who participated in the study were unaware of the concentration of solutions and only the biochemists who prepared the solutions in the laboratory of the Semnan University of Medical Sciences were informed of the encoding of solutions.
The instrument used to examine the pain included six areas: face remarks, crying type, breathing pattern, arm movements, foot movements and alertness, That score ranges from 0 to 7, zero represents the lowest pain and 7 are the maximum points and the most pain. In previous studies the validity of this instrument was 0.53 - 0.84 and its reliability was reported 0.92 - 0.97 (18, 20). In this study the reliability of the instrument was evaluated by simultaneous observation (r = 0.95).
For statistical analysis SPSS V. 17 software was used to determine the normality of the Kolmogorov-Smirnov test to determine the homogeneity of the groups from chi-square and Mann-Whitney and to compare the severity of pain in groups of Kruskal-Wallis, one-way ANOVA and Dunn’s test at the significant level 5% for data analysis.
4. Results
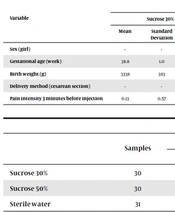
In this study 91 neonates in 3 groups, 2 case groups and 1 control group (31 neonates) were studied and the results showed that the groups did not different significantly in terms of gender, type of delivery, birth weight and gestational age, Demographic characteristics and mean pain severity for 3 minutes before injection of all three groups were summarized in Table 1.
| Variable | Control Group | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose 30% (N = 30) | Sucrose 50% (N = 30) | Sterile Water (N = 31) | ||||||||
| Mean | Standard Deviation | Percentage | Mean | Standard Deviation | Percentage | Mean | Standard Deviation | Percentage | ||
| Sex (girl) | - | - | 63.3 | - | - | 53.3 | - | - | 35.5 | > 0.05 |
| Gestational age (week) | 38.8 | 1.0 | - | 38.1 | 0.8 | - | 38.3 | 1.0 | - | |
| Birth weight (g) | 3338 | 383 | - | 3155 | 324 | - | 3412 | 363 | - | |
| Delivery method (cesarean section) | - | - | 60 | - | - | 73.3 | - | - | 74.2 | |
| Pain intensity 3 minutes before injection | 0.13 | 0.57 | - | 0.30 | 1.02 | - | 0.16 | 0.58 | - | 0.962 |
Immediately after injection, the mean pain intensity in the sucrose group 30%, 50% and sterile water was 3.63, 3.57, and 4.58%, respectively. The highest mean pain in the sterile water group and the lowest mean pain were in the sucrose 50% group. Comparison of mean pain intensity immediately after vaccine injection among the groups showed that the intensity of pain in infants who had received sucrose 30% and 50% was significantly different respectively (P = 0.047) and (P = 0.030) compared to sterile water.
Other results showed that the mean pain intensity of 5 minutes after injection in groups receiving 30%, 50% sucrose and sterile water had received respectively was 0.1, 0, and 1, so that the pain intensity in the sucrose group 30% (P = 0.001) and 50% (P < 0.001) was significantly less than the sterile water group. Comparison of concentrations of 30% and 50% sucrose with immediate and 5 minutes after injection did not show any significant difference (P > 0.05), this means that the analgesic effect of sucrose has not increased with increasing concentrations.
The mean and standard deviation of pain intensity 5 minutes after injection compared to immediately after injection of hepatitis vaccine in the studied groups are summarized in Table 2.
The results showed that the intensity of pain 5 minutes after vaccine injection was reduced in the case and control groups significantly in comparison with immediately after injection (P < 0.001).
| Samples | Immediately After Injection | After 5 Minutes Injection | P Value | |||
|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |||
| Sucrose 30% | 30 | 3.63 | 1.33 | 0.1 | 0.55 | < 0.001 |
| Sucrose 50% | 30 | 3.57 | 1.33 | 0.00 | 0.00 | |
| Sterile water | 31 | 4.58 | 1.67 | 1.0 | 1.53 | |
5. Discussion
In the present study the effect of 30% and 50% oral sucrose on relieving the behavioral pain responses of hepatitis B vaccine injection on 91 neonates showed that, sucrose 50% and 30% significantly reduced pain during immediately and 5 minutes after injection of the vaccine. But with increasing sucrose concentration, no significant increase was observed in its palliative effect. Many studies have not done the comparison of different concentrations of sucrose to a painful procedure.
A study conducted by Kristoffersen and colleagues in 2011 showed that 30% sucrose with a pacifier reduced the pain caused by the insertion of the nasogastric tube (21).
Curry et al. studied the effect of sucrose 50% and 75% on children of 2, 4, and 6 months who were vaccinated. For the assessment of pain, the physical manifestations scale (FLACC) and crying time were used. The results showed that there was no significant difference between pain score and crying time. Only in children who used sedation methods such as hug, shake and kick back to the baby, pain scale was significantly reduced (16).
The results of this study do not match with the present study but the study of Curry et al. was carried out by several researchers and in different clinics.
On the other hand, the samples were older in age. According to Schechter et al. many factors such as age, gender, previous painful experience, temperament and cultural context that cannot be altered impact in the experience of pain caused by immunization (22).
In another study that was conducted on the effect of sucrose (44%) on pain resulting from venipuncture in 0 - 6-month-old infants., the results showed that sucrose had no significant effect on pain score, crying time, and heart rate (23). It should be noted that this study is different from the type of procedure and the age of the samples and also in this study the FLACC (face, legs, activity, cry, consolability scale) score was used. The existing differences can justify the lack of coherence of the results of the two studies.
The study of Moradi et al. in Iran aimed at comparing the concentrations of 20% and 50% of sucrose to the pain caused by the hepatitis B vaccine showed that the effect of soothing sucrose did not increase with increasing concentration (17), which is completely consistent with the result of the present study.
In this study, the number of samples was low and pain intensity were measured only by the behavioral tools and it is suggested that in subsequent studies various too be used is that measure both physiological and hormonal responses to pain.
5.1. Conclusions
The results of this study showed that sucrose at a concentration of 30% and 50% reduced the pain associated with injection of hepatitis B vaccine in newborns, the analgesic effect of which is not associated with any increase in concentration. So it cannot definitely conclude that by increasing the concentration, analgesic effects also increase.
5.2. Application of Clinical Research Findings
In general using sucrose as a cheap and safe substance can reduce the pain associated with muscle injection in newborns and prevent complications caused by unrelieved pain