1. Background
Understanding the relationship between exercise, appetite, and weight management is necessary for sportsmen and women desiring to enhance their sports performance. Gut hormones (e.g., ghrelin) are potent regulators of appetite influenced by exercise (1). Moreover, appetite and food intake may be affected by factors such as sex (male or female) (2). Examination of exercise intensity on appetite-regulating hormones has only been investigated in highly-trained males (1). However, no report about the effect of progressive aerobic exercise training on the appetite-regulating hormone in females. Suppression appetite and subsequent food intake restriction in female athletes is higher than that of non-athletes and in those with Amenorrhea compared to normal menstrual (3, 4). Besides, energy reduction in female athletes can lead to a reduced IGF-1 level (5), performance decline (6), the prevalence of Amenorrhea (7), low energy availability, menstrual dysfunction, and decreased bone mineral density (4, 8). The mechanisms by which exercise suppresses appetite temporarily are not fully understood but may involve a reduction in ghrelin concentration (2). Ghrelin is a 28-amino acid peptide, which is mainly secreted from the stomach and leads to an increase in appetite and secretion of growth hormone (9). Two types of ghrelin (Acylated Ghrelin and Des-acyl Ghrelin) have been identified in the circulatory system, named total ghrelin (9). Des-acyl Ghrelin cannot affect appetite (10). So, it seems that the assessment of total ghrelin is not a precise index for appetite. It has been suggested that the measurement of acylated ghrelin could be a more accurate method (11, 12). Some studies have shown that serum levels of acylated ghrelin reduce after endurance running and resistance training (13, 14). Recently, Gorzi et al. (15) reported a decrease in acylated ghrelin in the stomach tissue after strenuous interval exercises. However, Haghshenas et al. (16) demonstrated that eight weeks of endurance training had no effects on the plasma levels of acylated ghrelin. On the other hand, Rashidlemir et al. (17) showed that eight weeks of aerobic training in young men increased acylated ghrelin and decreased plasma growth hormone levels significantly. Similarly, 12 weeks of high strenuous training increased acylated ghrelin levels in rats (18). Also, eight weeks of aerobic training at 65% - 68% of maximum heart rate increased ghrelin concentration of healthy females (19). On the other hand, the suppression of ghrelin secretion after exercise can lead to appetite decline and reduces food intake. This decrement over a long-time period would cause negative energy balance and subsequent body weight loss (2). There are controversial reports about the effect of exercise on appetite (2, 7, 13, 14, 16, 18, 20-25) and some closely related topics, such as a change in IGF-1 (5, 26-28), inadequate food energy, and negative energy balance (2), especially in menstrual conditions (4).
2. Objectives
Therefore, the purpose of this study was to investigate the effect of long-term endurance running on gastric tissue and serum acylated ghrelin, the serum level of IGF-1, and weight changes in female rats.
3. Methods
3.1. Animals
Female Wistar rats (200 - 220 g) were purchased from the Pasteur Institute of Iran. They were placed on a 12/12-h light-dark circuit in a temperature (23 ± 2°C), and humidity (%45 ± 5) controlled room. Food and water were provided ad libitum. All rats initially were familiarized with running on the treadmill at the speed of 5 m/min for 10 minutes in the first week. After the acclimation period, to control the intervening variables such as menstrual cycle synchronization and body weight differences, animals were randomly assigned to two groups (control and PAT, n = 8).
3.2. Aerobic Exercise Protocol
The exercise training was performed on a motorized treadmill. The training program consisted of 12 weeks of aerobic running (Five sessions per week at 8 AM - 10 AM). The progressive aerobic exercise on the treadmill started at 13 m/min for 15 min with a zero-degree slope in the first week and reached up to 25 m/min for 55 min in the fourth week (16). This exercise program lasted for eight weeks. Each training session included warming up (5 min at the speed of 10 m/min), main program (duration up to 55 min at the speed of 10 - 25 m/min), and cool down (5 min at the speed of 10 m/min), respectively (29).
3.3. Ethical Considerations
All experimental procedures were under the guidelines for the care and use of laboratory animals published by the National Institute of Health (NIH) and approved by the Animal Ethics Committee of the University of Zanjan.
3.4. Blood and Tissue Sampling
The rats were sacrificed 48 hours after the last training session to rule out the acute effects of exercise. Blood samples were taken from their heart and centrifuged for 10 min with 3000 rpm. Also, the stomach was dissected, and the fundus that has high ghrelin hormone levels was removed to measure ghrelin (30, 31). The samples were washed in the saline solution and immediately frozen in liquid nitrogen and stored at -80°C until laboratory measurements. The tissues were homogenized in PBS buffer. The homogenate was centrifuged for 15 min at 3,000 rpm, and its supernatant was used to measure the ghrelin (15).
3.5. Measurement of Serum and Gastric Acylated Ghrelin and Serum IGF-1 Levels
The concentration of serum and gastric acylated ghrelin hormones were measured using ELISA specific kits for rats (ECPlaza Co., Bioassay TL, China); based on the kit manufacturer's guideline (kit sensitivity was 0.05 ng/L). IGF-1 was measured by the ELISA method using the China Bioassay Laboratory Kit with a sensitivity of 1.55 ng/mL.
3.6. Data Analysis
All results were expressed as mean ± standard deviation (SD). Normality was verified using the Shapiro-Wilk normality test. After ensuring the normal distribution, the independent t-test was conducted to assess the difference between the two groups. The significance level was set at P < 0.05. All statistical analyses were performed using SPSS (version 16).
4. Results
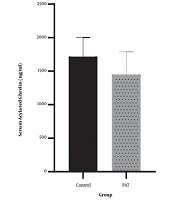
4.1. Serum Acylated Ghrelin
The results indicated that 12 weeks of PAT (1450.3 ± 341.1) had no significant effect on serum acylated ghrelin (P = 0.15, t = -1.54) compared with the control group (1716.8 ± 286.5). The results are fully described in Figure 1.
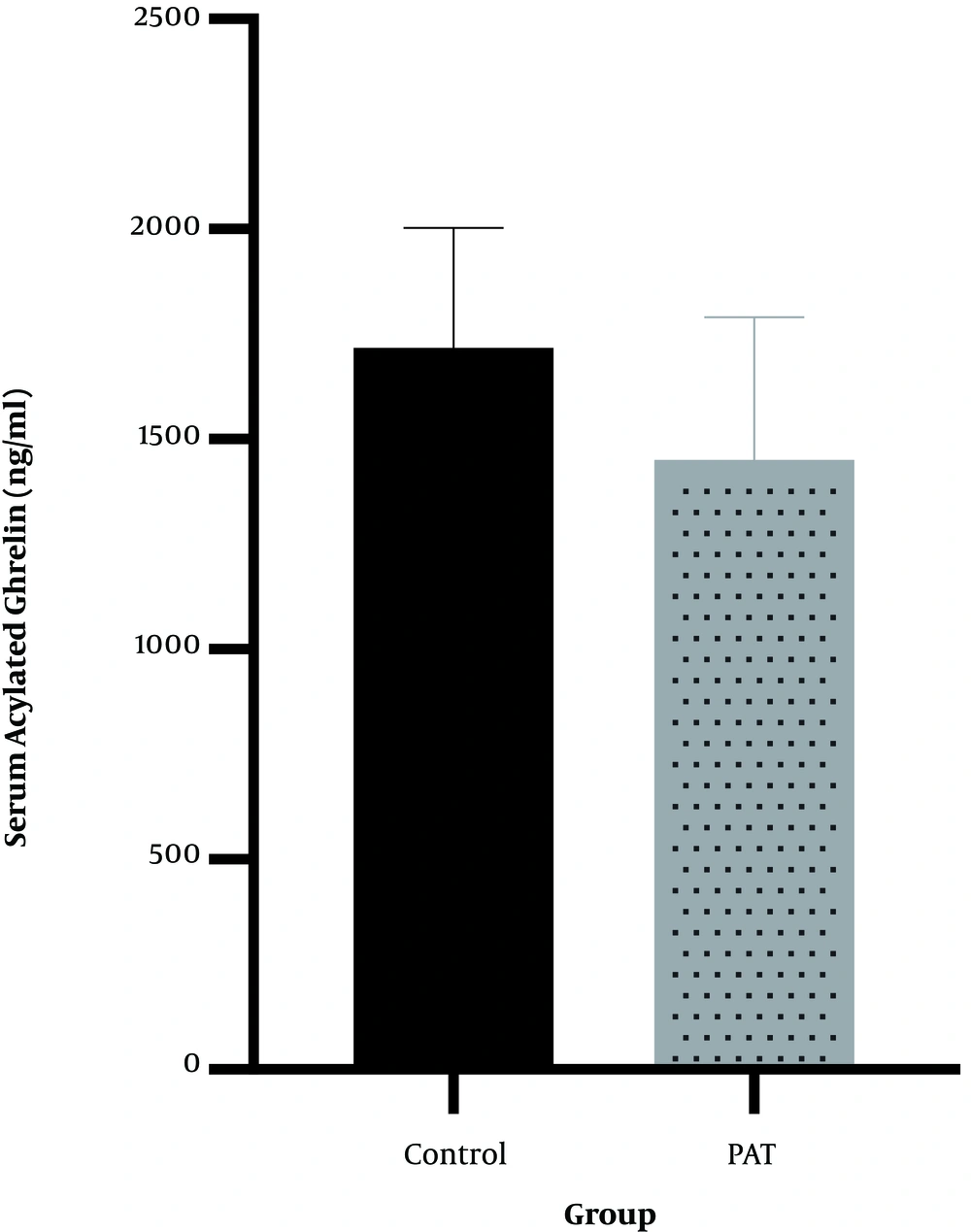
4.2. Gastric Tissue Acylated Ghrelin
The results indicated that 12 weeks of PAT (708.6 ± 274.0) had no significant effect on gastric tissue acylated ghrelin (P = 0.51, t = 0.686) compared with the control group (690.25 ± 85.7). These results are shown in Figure 2.
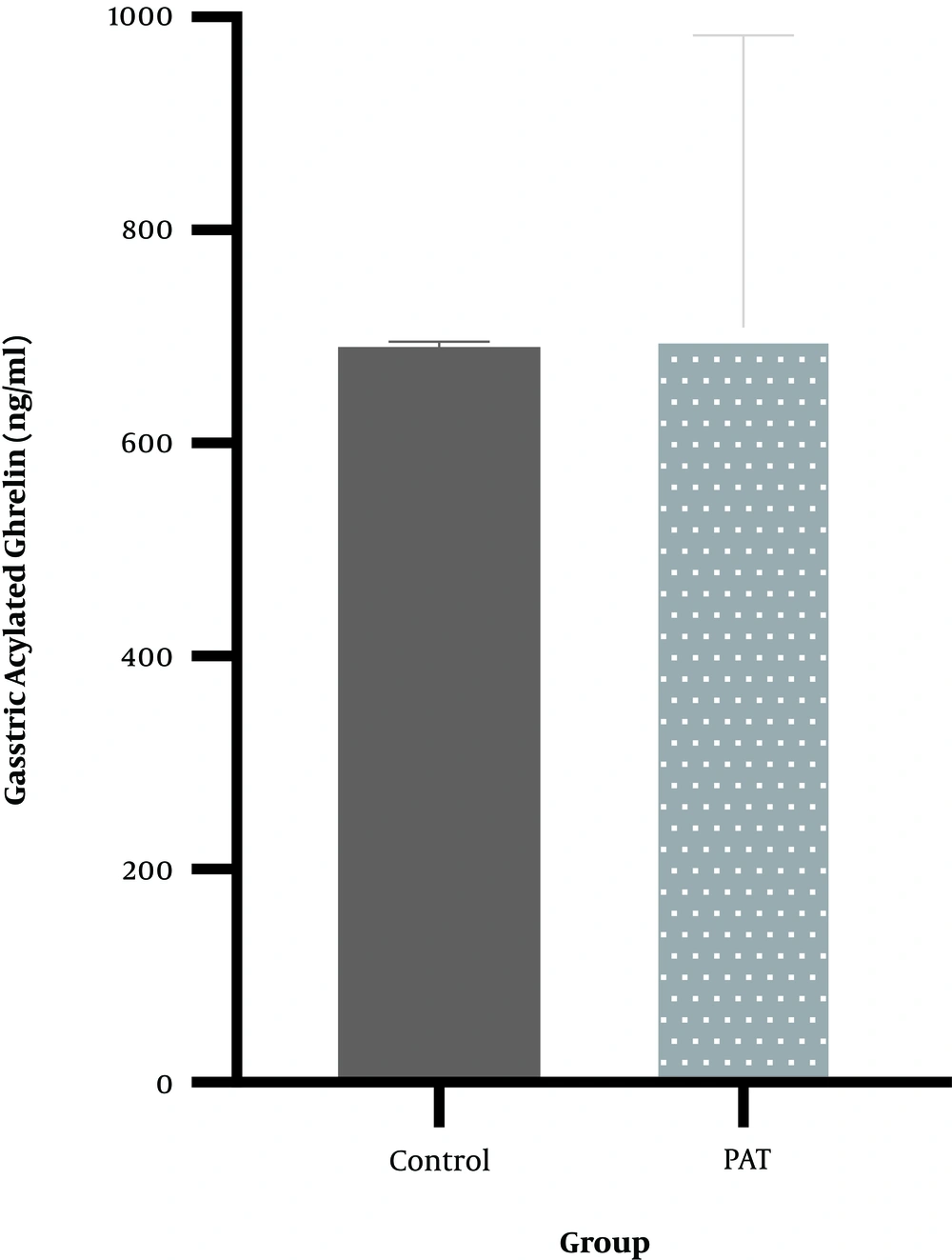
4.3. Weight Changes
As shown in Figure 3, twelve weeks of PAT (21.50 ± 5.12) had no effects on weight changes (t = -0.825, P = 0.42) compared with the control group (24.87 ± 10.37).
Also, feed intake was measured. There were no significant differences in the mean food consumption between the control (676 ± 118 g) and PAT (675 ± 111.02 g) groups.
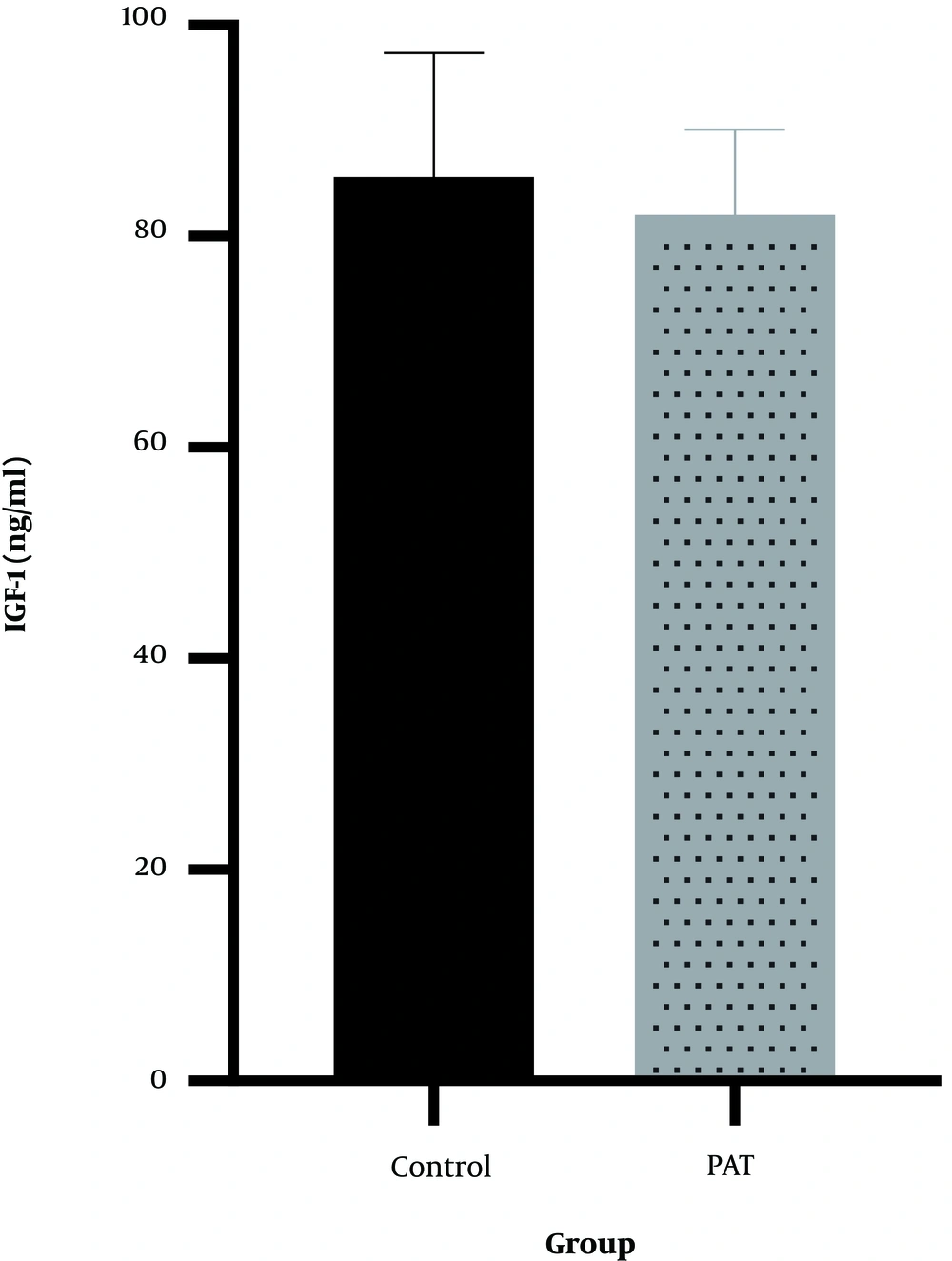
4.4. Serum IGF-1
The results showed that 12 weeks of progressive aerobic exercise (82.0 ± 8.1) had no effects on serum IGF-1 (P = 0.56, t = -0.598) compared with the control group (85.6 ± 11.8) (Figure 4).
5. Discussion
The results indicated that 12 weeks of PAT had no significant impact on body weight, gastric acylated ghrelin, serum acylated ghrelin, and IGF-1. Reduction in acylated ghrelin following exercise has been reported by some studies (13, 14). Although ghrelin levels decrease after exercise, its effects persist up to several hours following exercise and can lead to a reduction in appetite. Consequently, this causes a negative energy balance and loss of body weight (2). Hence, it seems that the results of this study are substantial; As they show that appetite hormones such as acylated ghrelin are not efficiently reduced due to a relatively long period of PAT.
Our results are inconsistent with the findings of Hagshenas et al. (16), who observed a significant reduction in the weight of the male rats following an exercise program. Similarly, some studies have shown that appetite suppression occurs after moderate to high-intensity exercise (32, 33). In contrast, Fathi et al. (18) reported that high-intensity exercise increases acylated ghrelin, which is also inconsistent with the results of the present study. Despite the high-intensity exercise in this study, there was no increase in acylated ghrelin levels. The main difference between the current research and other studies is the implementation of female rats instead of male ones. It seems that the response of female rats to high-intensity aerobic exercise is different from that of male animals. Hagobian and Braun (34) showed that the hormonal and appetite responses to exercise are strongly influenced by energy balance in men, but much less so in women, because physical activity affects the energy intake side of the energy balance equation, and this effect differs by sex. Accordingly, Potteiger et al. demonstrated that 16 months of aerobic exercise decreased body weight and fat mass in men who ate ad libitum, whereas no changes were observed in women (35). Likewise, Oscai et al. (36) reported that female rats that swam for six hours daily, six days/week, gained weight at the same rate as sedentary freely eating, while male animals gained weight significantly more slowly than sedentary controls. So, it seems that there is a sex-dependent appetite response to progressive endurance running.
Further, evidence shows that only 19% of the intervention studies report an increase in energy intake after exercise, 16% show a decrease and 65% show no change in appetite (as cited in Vatansever-Ozen) (37). Appetite regulation can be affected by decreases in acylated ghrelin and increases in PYY, GLP-1, and pancreatic polypeptide. Moreover, blood redistribution during exercise may be important for suppressing ghrelin, while other mechanisms involving cytokine release, changes in plasma glucose and insulin concentrations, (38) as well as tonic peptides such as leptin (39). However, the specific action of exercise on each physiological component will vary in strength from person to person (according to individual physiological characteristics) and with the intensity and duration of exercise (39). On the other hand, the exercise-induced elevation of ghrelin is not long-lasting. For example, in a study, the plasma ghrelin levels were measured 4 hours after high-intensity interval exercise to compare with the sedentary group (40). So, since we sacrificed the animals at 48 hours after the last exercise session, it is likely that ghrelin concentration has returned to baseline levels.
It has proposed that the ghrelin system normally enhances exercise endurance by increasing IGF-1 levels and/or increasing the availability of gluconeogenic substrates such as lactate to meet the energy demand of prolonged exercise (40). Interestingly, our results indicated that the exercise program had no significant effect on serum IGF-1. So, unchanged levels of IGF-1 in the current study can be attributed to no significant change in ghrelin levels. Similarly, some studies have shown unaltered levels of IGF-I after endurance or resistance training (5, 41, 42). In addition, training-induced increases in IGF-1 could occur in muscle without changing serum IGF-1 levels (6). Sheykh Aleslami Vatani and Bordbar (43) showed that resistant training could increase strength without significant altering in serum IGF-1 concentration. Furthermore, skeletal muscle-derived IGF-I that contributes to total circulating IGF-I levels increases at high levels 5 - 10 min after the start of moderate to strenuous exercise and rapidly clears from the blood (44). In the present study, we sacrificed the animals 48 hours after the last training session; maybe, during this period, the initial increase in IGF-1 has returned to its average level.
On the other hand, it has been shown that strenuous exercise exposes women athletes to IGF-1 drop (5), performance decline (6), energy loss, menstrual dysfunction, and reduced bone density (4, 8). So, it seems that the exercise program in the present study probably had no enough intensity to challenge the endocrine axis seriously; therefore, IGF-1 levels remained unchanged compared to the control group.
However, our ability to interpret some of the findings would have been improved if we had measured other appetite hormones, body composition, and sex-related hormones affecting the menstrual cycle. Also, the current study did not include male subjects; so, we could not compare males and females. In addition, the lack of menstrual cycle synchronization was another limitation in our study. Finally, acylated ghrelin and IGF-1 were measured at only 48 hours after the last exercise session. More frequent measurements could provide more precise perspective in this context. Certainly, there is a need for more comprehensive studies to better understand the effect of exercise on appetite and related hormones in females.
5.1. Conclusions
Our results, and that of other studies, show that exercise-induced changes in IGF-1, body weight, serum acylated ghrelin, and gastric tissue acylated ghrelin, may be sex-dependent. These sex-based differences can imply both basic science and practical application. However, understanding the mechanism of different female-specific hormonal and appetite responses to progressive aerobic exercise requires more research studies at several scales.