1. Background
The prevalence of gallstones has significantly increased, such that only in the US about one million cases are diagnosed each year (1). This disease imposes a significant therapeutic-economic burden on the healthcare systems, even in western countries, to the extent that between 10% - 20% of people in the European and American societies are suffering from gallstones (2, 3) and refer to hospital emergency rooms with acute abdominal pains (4). In most cases, gallstones symptoms are not significant. As about 25% - 50% of those suffering from gallstone develop several complications, it is necessary to remove their gallbladder (5) using surgical techniques such as laparoscopic cholecystectomy, which is highly common. Laparoscopic cholecystectomy is one of the most common abdominal surgeries and the standard treatment for gallstones and cholecystitis (6). In addition, it is a non-invasive technique (7) intended to reduce the potential damages of the disease (8). Apart from several advantages, laparoscopic cholecystectomy is associated with better hemostasis compared to open surgeries (9). Due to several reasons, including the need for a small incision, low pain, short duration of hospitalization, early recovery, early start of eating food, and, in general, the quick return to daily activities, the laparoscopic cholecystectomy is the preferred option to treat most of the cases who suffer from the gallbladder (10, 11). According to the currently available evidence, about 0.04 of cases who underwent the laparoscopic cholecystectomy develop complications (12) such as bile duct injury, acute biliary tract obstruction, extrahepatic biliary duct rupture, falling gallstones into the abdominal cavity, and the subsequent formation of an abscess. Moreover, it also may cause some intraoperative complications, such as hemodynamic changes during blowing the gas into the peritoneum, including cardiac output, increased systemic vascular resistance, hypertension, heart rate changes, and reduced respiratory capacity (13). In addition, it may cause some changes in the body, such as changes in acid-base, pulmonary status, cardiovascular system, and hemodynamic. There are reported that attributed changes in the liver function to impaired portal vein flow, decreased venous flow, and variations in intracranial pressure (14). A dysfunction in the blood flow to the liver not only may result in liver dysfunction but also can disrupt the production of proteins made in hepatocytes, including coagulation factors (PT, PTT, and INR) (15). Some empirical evidence reported PT and PTT levels following laparoscopic cholecystectomy (15, 16); however, the evidence are still inconclusive over changes in the INR factor (16).
2. Objectives
Given the high prevalence of gallbladder diseases and the tendency to undergo laparoscopic surgeries, the present study aimed to investigate the effect of laparoscopic cholecystectomy on coagulation tests among patients undergoing the surgery at Ali ibn Abi Taleb Hospital in Zahedan (Iran). It is hoped that the findings of this study be a step towards solving the problems with which these patients have to deal.
3. Methods
3.1. Research Design
The current study was carried out following a quasi-experimental design and using a convenience sampling method (for screening).
3.2. Statistical Society and Participants
The statistical society of the present study included those scheduled for laparoscopic cholecystectomy, for whatever reasons, at Ali ibn Abi Taleb Hospital in Zahedan. A total of 21 cases were recruited.
3.3. Inclusion and Exclusion Criteria
The inclusion criteria, which were determined based on previous studies, were as follows: all patients with symptomatic gallstones who based on sonography findings had stones in their gallbladder, being aged 18 to 75 years, a maximum body mass index (BMI) of 40, normal levels of PT, PTT, and INR markers, and being ready to undergo the surgery (13). The exclusion criteria were patients’ intolerance to general anesthesia, having irrevocable coagulation disorders, suffering from metastatic lesions, and those whose surgery was changed from laparoscopic to open surgery during the surgery (17).
3.4. Experimental Design
The present study was carried out in a way that, before the surgery, a venous catheter was considered for the patients, and their anesthesia was initiated with a dose of propofol (2 - 3 mg/kg) and fentanyl (0.5 g/kg) in the first 5 minutes, and it continued with 200 - 250 µg/dL of propofol per minute. Then, the orotracheal intubation was performed. During the surgery, their applicable ringer’s solution was 20 cc/kg. The surgery was conducted using the four-port technique, i.e., a 10-mm umbilical port, which was the first port that entered the abdomen with the aid of aport needle after umping the gas into the abdomen, a 10-mm xiphoid port, a 5-mm mid-clavicular port at the Murphy point, and a 5-mm mid-clavicular port in the adjacency of umbilicus. During the surgery, the intra-abdominal pressure was maintained by a maximum of 15 mmHg of carbon dioxide, and the patients were under continuous cardiac and capnography monitoring. The patients’ blood samples were collected at three different time points, i.e., before the surgery (time point 1), 30 minutes after pumping the carbon dioxide gas into the abdomen (time point 2), and 30 minutes after removing the last port from the abdomen (time point 3). The blood samples taken in these time points were examined concerning daily PT, PTT, and INR serum levels and then were transferred to a laboratory under a cold chain for further investigations (14). It should be noted that the current study was carried out without a sexual preference (women), and steps mentioned in the anesthesia service reviews and the protocol of the American Society of Anesthesiologists were followed; hence, no preoperative preventive measure was taken. Furthermore, there were no indications for the intraoperative injection of low-molecular-weight heparin (LMWH) and antibiotics.
3.5. Statistical Analysis
Data analysis was performed using SPSS Ver.22 by repeated measures and Bonferroni follow-up test.
4. Results
4.1. The Effect of Laparoscopic Cholecystectomy on Coagulation Tests Among Patients Undergoing the Surgery
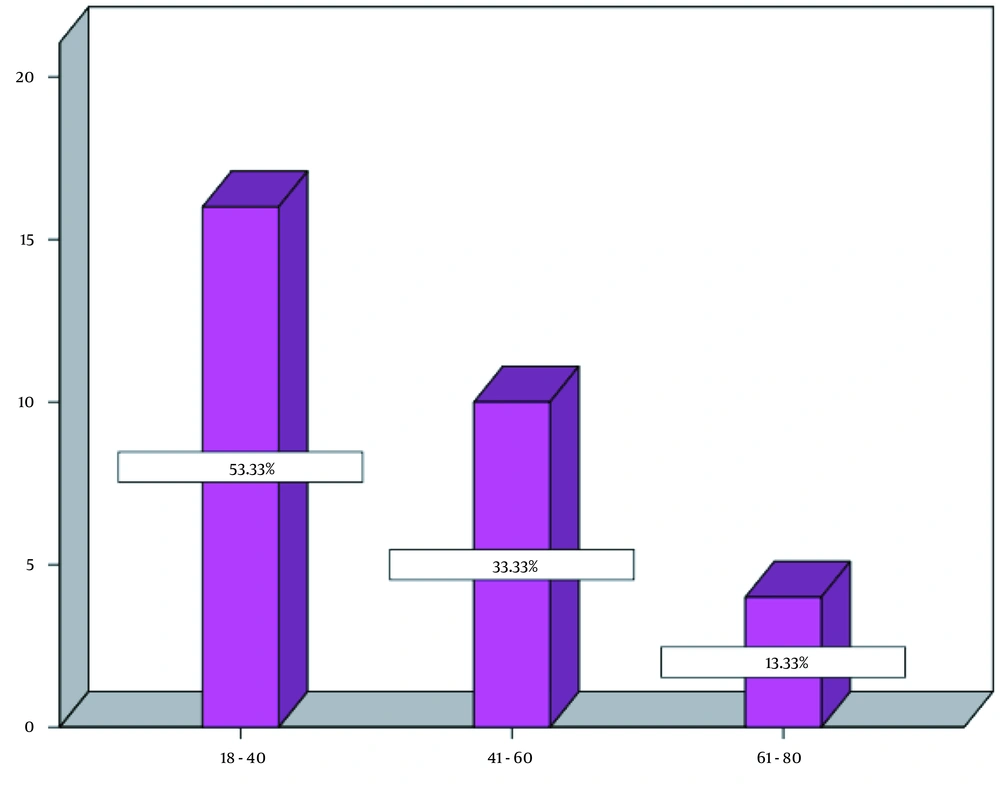
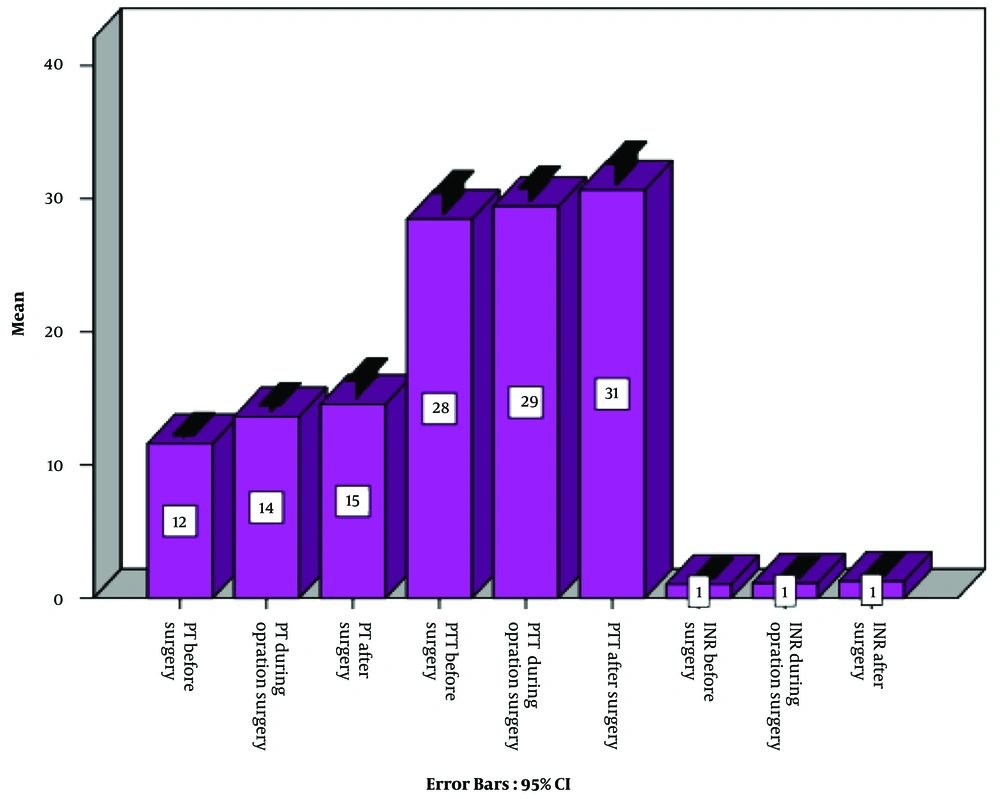
Before examining the research hypotheses, The Kolmogorov-Smirnov test was applied to test for a normal distribution, which indicated the normality of all variables, as the significance values of the normality tests were greater than 0.05. In addition, the assumption of equal variances was examined for each variable by Levene’s test, which resulted in significant levels for all investigated variables. Hence, the assumption of equal variances was observed. Evaluating the descriptive findings on demographic characteristics indicated that 53.3% of patients aged 20 to 40 years, 33.3% aged 41 to 60 years, and 13.3% aged 61 to 80 years (Figure 1). In general, most of the respondents were aged 18 to 40 years old, and the mean operative duration was 54 minutes. Investigating descriptive findings (Figure 2) related to the main research variables demonstrated significant changes in coagulation factors (i.e., PT, PTT, and INR) in the second and third points of time compared to the first point; nevertheless, considering the inferential findings obtained by analyzing the research hypotheses (Table 1), it can be argued that according to the repeated-measures statistical analysis of variance conducted by Wilks’ Lambda test, despite increased mean values of the coagulation factors (PT, PTT, and INR) in the second and third points of time in comparison with the first point, only PT (EF = 28.657; P = 0.0005) was significant at the 99% confidence level and indicated a difference amongst these three points. In other words, it can be stated that the laparoscopic cholecystectomy surgery only had a significant effect on the changes in the PT factor, and these changes were statistically significant comparing the first and second points of time and the first and third points of time; however, since the INR level did not change, the changes in PT could not be assessed. Hence, they were not clinically significant.
| Factor | Time 1, Mean ± SD | Time 2, Mean ± SD | Time 3, Mean ± SD | P-Value (Comparing Time 1 and 2) | P-Value (Comparing Time 1 and 3) | P-value (Comparing Time 2 and 3) |
|---|---|---|---|---|---|---|
| PT | 11.610 ± 1.0060 | 13.63 ± 4.29 | 14.57 ± 3.963 | 0.0005 | 0.001 | 0.273 |
| PTT | 28.47 ± 4.524 | 29.43 ± 2.91 | 30.67 ± 4.528 | 0.907 | 0.146 | 0.176 |
| INR | 1.043 ± 0.1382 | 1.117 ± 0.2036 | 1.263 ± 0.6344 | 0.080 | 0.162 | 0.348 |
The Results of the Bonferroni Follow-up Test Intended to Investigate the Effect of Laparoscopic Cholecystectomy on the PT
5. Discussion
This study demonstrated that the laparoscopic cholecystectomy surgery was only effective in changing (i.e., increasing) the PT coagulation factor and did not have any significant effects on other coagulation factors. Similar results are reported by Garg et al. as cited in Donmez et al. (18), which indicated that this surgical technique led to an increase in the level of PT. However, different results are reported by Papaziogas et al. (17). In explaining this finding, increased mean PT following laparoscopic cholecystectomy surgery can be attributed to the activity of the patients’ coagulation systems, and the fact that coagulation proteins and the laparoscopic technique may cause a highly severe catabolic liver response to the liver after the surgery (15). Additionally, the change in the PT factor is related to the laboratory kit, and the lack of change in the INR level is consistent with the results of a study carried out by Marakis et al. (19). Concerning the relative stability of the INR level, it can be argued that the laparoscopic cholecystectomy can reduce the activation of the hemostatic mechanism, such that in the event of clotting, stimulates the fibrinolytic system and causes the release of a proteolytic enzyme called plasmin. Subsequently, this enzyme breaks down some of the bonds in the fibrin molecule and results in the release of peptide products and, thus, leads to the dissolution of the clot and results in the relative stability of the INR level (17, 19). Generally, it can be stated that although some variations occurred in the PT coagulation factor during the laparoscopic surgery, these changes did not affect the INR level. However, it should be noted that laparoscopic cholecystectomy surgery is safe with regard to renal and liver functions. It is worth noting that pneumoperitoneal pressure has a relative effect on coagulation and fibrinolysis during laparoscopic cholecystectomy surgery. In the current study, increased PT level can not be attributed to intra-abdominal gas pressure, which is in line with the studies by Donmez, et al. (18) and Garg et al. (20); nevertheless, based on previous studies (18, 20-22), it can be attributed to its association with pneumoperitoneum, so that both pressures of 10 mmHg and 14 mm pneumoperitoneum may affect coagulation tests and fibrinogen levels. Some studies reported that only pressure of 10 mmHg could decrease the PT level, while, on the other hand, pressures higher than 14 mmHg could increase the PT level. Moreover, applying 9 to 17 mmHg, there may not even be any effects of pneumoperitoneum and the type of gas on the internal factor. Therefore, it has a more negative effect on coagulation and fibrinolysis cascade than low pressures (18). Thus, except for the impacts of pneumoperitoneum on portal vein flow due to liver functions and their related inflammatory responses, some intervening factors, such as age, BMI, surgery duration, and surgical injuries, affect (either directly or indirectly) can change the PT levels. Accordingly, patients with risk factors such as senescence, obesity, or a long wait for laparoscopic surgery, are likely to have significant coagulation activations. They are considered a high-risk group who are prone to the development of deep vein thrombosis following the surgery, which certainly causes a kind of thromboprophylaxis. In this regard, laparoscopic surgery activates the coagulation and bronchiolitis pathways and changes the height of the bTG and coagulation tests. It is necessary to mention some limitations and biases of our study. Firstly, not having the opportunity to assess the functions of the pancreas and other organs in the abdomen during the surgery, due to its high cost. Secondly, not having the chance to examine the functions of the organs after the surgery to evaluate postoperative changes. Thirdly, all participants of the present study were females who none of them was admitted to public hospitals.