1. Background
Today, cardiovascular diseases are the most common cause of death. They are considered the primary cause of about 40% of death numbers in developed and undeveloped countries (1). Although there is no sufficient information on the starting point of arrhythmia, sinus tachycardia frequently happens after acute heart stroke (2). In acute heart stroke, approximately all rhythm abnormalities like tachycardia and bradycardia are the most common. Heartbeat irregularities are heart diseases, including bradycardia (slow beating) and tachycardia (fast beating), which may culminate in abnormal heart rhythm. One of the most dangerous side effects following an acute heart stroke is tachyarrhythmia (3). Cardiovascular diseases are caused by various factors such as high blood pressure and incorrect lifestyle (4). High blood pressure is among the risk factors of cardiovascular diseases. Hypertension has no symptom until it is completely developed. It augments the heart beating level and weakens heart muscles (5).
On the other hand, the lack of physical activity is another risk factor for cardiovascular diseases. The effects of aerobic exercise on blood pressure in long period exercise planning and increased exercise sessions are frequently studied (6). Many studies have examined the effects of various exercise models on systolic and diastolic blood pressure, and heart rate, either directly or indirectly. Most of research has focused on the effects of exercises on healthy and athletic individuals (7-9). We did not find any research that exclusively examines the effects of various exercises in patients with arrhythmias. Various forms of exercise are given great importance, among which the Pilates and walking have been the most attractive ones. The Pilates is a collection of professional activities in which strength, balance, and flexibility are the most fundamental elements and cooperates body, mind, and soul. The Pilates focuses on the movement of muscles and joints, which are frequently involved in daily activities like sitting, walking, bending, and erecting (10). Also, Pilates improves the pulmonary function index (11).
2. Objectives
As no comprehensive study examined the effects of the exercise on heart arrhythmia, our study tries to answer the following question: can eight weeks of Pilates, walking, and combined Pilates and walking induce significant effects on resting heart rate, resting systolic and diastolic blood pressure of patients with tachycardia?
3. Methods
The present study was done in a quasi-experimental model with a pre-test and post-test design. The purpose of this study was to investigate the effects of Pilates, walking, and combined Pilates and walking exercise for eight weeks on resting heart rate (RHR), resting systolic, and diastolic blood pressure in patients with arrhythmia. Statistical population includes the patients with tachycardia in Zahedan in 2018. The statistical sample consists of 30 females, aging 24 to 59 years old. The exclusion criteria were the patients had tachycardia, having no regular exercise during six last months, having no cardiac surgery, having no knee joint operation, intervertebral disc, and neck operation. The participants were selected purposefully and were categorized into three distinct groups: Pilates (N = 10), walking (N = 10), and combined Pilates and walking (N = 10). In the beginning, an explanatory meeting was held to clarify research process and possible profits and dangers of the study and to let the participants complete a form, including their personal information and their satisfaction to participate in the study procedure. The participants were assured that all their personal information would be confidential, and they were allowed to quit the study as soon as they wish. The participants’ RHR was measured by ECG electrocardiograph, Yasham 310, Germany. Resting systolic and diastolic blood pressure was measured by an AccuMed digital gauge in three different steps. The first measurement was performed 24 hours before starting the exercise, the second one at the end of the fourth week, and the last one was performed 24 hours after the end of the eighth week. In each step, the participants relaxed for 10 to 15 minutes before the measurements. To determine the severity of activities (60% to 70 % maximal heart rate), we used the maximum heart rate formula (12).
3.1. Pilates Protocol
Pilates exercise was performed for 60 minutes, three times a week for eight weeks. It consisted of some simple movements, which mostly involved body muscles, gluteal muscle, psoas muscle, and pelvic floor muscle. Actions were done on a bed in standing up, lying down, and sitting states. Activities were started from plain movements and were gradually extended. Whenever it was necessary, the movements needed some specific balance were changed. To follow the overload principle, increasing the movement repetition played incrementally in a way that they were started from 7 and ended to 20. Every session was started with 15 minutes warming up stretching movements, continued with 30 minutes Pilates and was finished with 15 cooling down movements. After consulting the trainer and the researcher, Pilates movements were selected in a way to impose less pressure on the participants’ cardiovascular system. The intensity of exercise was from 60% to 70% of maximum heart rate (13). To control the intensity of exercise, an accurate pulse oximeter, Germany, was used.
3.2. Walking Protocol
The walking process was just performed on a treadmill to control the condition carefully. Therefore, corrected Bruce protocol was applied. This protocol, except its first two steps, was the same as Bruce’s. The first step was started with a 0-degree slope and 1.7 miles/hour speed (0 to 3 minutes). In the second step (4 to 6 minutes), the slope was increased by 5%. The third step was continued with a 10% slope and 1.7 miles/hour speed while the slope and the rate were increased to 12% and 2.5 miles/hour in the fourth step. The fifth step was ended in a 14% slope and 3.4 miles/hour speed (14). Although the protocol has twelve steps, the specialist suggested continuing just up to the fifth step with a 14% slope. At the end of the eighth week, only time and speed were added. Doing exercise was continued as far as the participants were able to go on.
3.3. Combined Pilates and Walking Protocol
The participants in the combined Pilates and walking group first did some Pilates exercise and then started walking on the treadmill. The Pilates prolonged for 60 minutes; then the training was continued with walking on a treadmill as far as the participants declared their tiredness and inability to continue.
3.4. Statistical Methods
The results were analyzed using the Kolmogorov-Smirnov test. To test the research hypothesis, one-way ANOVA and repeated measure ANOVA were applied. Bonferroni post hoc test was applied to determine differences among the groups. Data were analyzed by SPSS software version 20.
4. Results
The results demonstrated a significant reduction of RHR at the end of eighth Pilates exercise compared to the beginning of the research. This considerable reduction was observed in the walking and combined walking and Pilates group (Table 1).
| Groups | Mean ± SEMa | P Value | ||
|---|---|---|---|---|
| Pilates | 1st week | 4th week | 6.4 ± 1.343 | 0.003* |
| 8th week | 16.6 ± 3.317 | 0.002* | ||
| Walking | 1st week | 4th week | 2.8 ± 0.800 | 0.020* |
| 8th week | 6.4 ± 1.802 | 0.019* | ||
| Combined training | 1st week | 4th week | 5.7 ± 1.850 | 0.039* |
| 8th week | 10.1 ± 1.602 | 0.001* |
aThe mean difference in sampling time in this study.
In comparison to the first week, resting systolic blood pressure at the end of eighth week showed a significant reduction in the Pilates (P = 0.001), walking (P = 0.01), and combined Pilates and walking groups (P = 0.021), and the comparison of the results in the fourth week with the first one showed a significant reduction of resting systolic blood pressure (Table 2).
| Groups | Mean ± SEM | P Value | ||
|---|---|---|---|---|
| Pilates | 1st week | 4th week | 13 ± 3.35 | 0.011* |
| 8th week | 13 ± 2.001 | 0.001* | ||
| Walking | 1st week | 4th week | 7.50 ± 2.713 | 0.066 |
| 8th week | 11 ± 2.769 | 0.010* | ||
| Combined training | 1st week | 4th week | 2.5 ± 2.007 | 0.733 |
| 8th week | 10.5 ± 3.023 | 0.021* |
As it is demonstrated in Table 3, the participants’ resting blood pressure in the walking group and combined Pilates and walking group showed a significant reduction after eight weeks. This reduction was evident in both walking and combined Pilates and walking groups in the fourth week in comparison to the first week.
aP < 0.05.
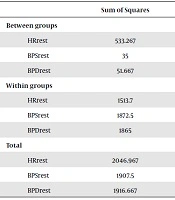
To study average variances among different studied groups, one-way ANOVA was applied. The difference between post-test and pre-test in the studied groups was calculated (gain scores), to control the possible effects of the pretest results on post-test ones. Then, one-way ANOVA was performed. Testing results showed a significant difference in heart rate among the studied groups (P = 0.017). One-way variance analysis of resting systolic and diastolic blood pressure of patients with tachycardia after eight weeks did not result in a significant difference among the studied groups (Table 4).
| Sum of Squares | df | Mean Square | F | P Value | |
|---|---|---|---|---|---|
| Between groups | |||||
| HRrest | 533.267 | 2 | 266.633 | 4.756 | 0.017* |
| BPSrest | 35 | 2 | 17.500 | 0.252 | 0.779 |
| BPDrest | 51.667 | 2 | 25.833 | 0.374 | 0.691 |
| Within groups | |||||
| HRrest | 1513.7 | 27 | 56.063 | ||
| BPSrest | 1872.5 | 27 | 69.352 | ||
| BPDrest | 1865 | 27 | 69.074 | ||
| Total | |||||
| HRrest | 2046.967 | 29 | |||
| BPSrest | 1907.5 | 29 | |||
| BPDrest | 1916.667 | 29 |
Abbreviations: BPDrest, resting diastolic blood pressure; BPSrest, resting systolic blood pressure; HRrest, resting heart rate.
As Levene’s test showed, homogeneity of variances was not fulfilled (P = 0.003). To compare the average in different groups, post hoc Dunnett’s T3 was applied. The results show that RHR in the Pilates group decreased significantly in comparison to the walking group after eight weeks (P = 0.049). Although this reduction was also seen in the combined Pilates and walking group, it was not significant (P = 0.262).
5. Discussion
The results of the present study showed eight weeks of Pilates, walking, and combined Pilates and walking exercise in patients with tachycardia demonstrated a significant reduction in heart rate. It was also shown that systolic and diastolic blood pressure decreased either, but it was not significant. The results showed that Pilates could induce a significant reduction in resting heart rate in comparison to the walking group. The results of the Gui’s study showed that intermittent exercise significantly reduced the blood pressure of patients with hypertension (15). In some studies, it has been reported that mean and intense physical activities, both will lead to heart rate reduction (15, 16). Another study showed that walking caused a reduction of resting heart rate in patients with chronic heart failure (17). Oh et al. in a study indicated that severe exercise culminates in the reduction of heart rate and systolic blood pressure (18). Also, some research studied the effects of walking on heart rate before and after exercise in senior women, which showed that walking could reduce the heart rate (19). In a study done by Williams and Franklin in two walking and running groups, it was observed that the regular exercise lessened the dangers of Arrhythmias (20). Michael et al. concluded that heart rate during an exercise was managed by both sympathetic and parasympathetic nerves (21). Cunha et al. reported that RHR oscillations depended mainly on sympathetic and vagal modulatory influences, but after maximal exercise, when parasympathetic contribution is negligible, the fast recovery is dependent on vagal reactivation with a later influence of both vagal reactivation and sympathetic withdrawal. Moreover, vagal recovery is improved with greater availability of acetylcholine by anticholinesterase inhibitor administration (22). The procedure in which exercise improves heart rate is not known, but it is generally accepted that exercise will increase nervus vagus and decrease the sympathetic effect of the heart (23). It seems physical activities will reduce the stability against bloodstream and reduce heart rate during resting through increasing the number of capillaries inactive skeleton muscles (24). Heart rate is managed by the sympathetic and parasympathetic system in a way that any failure in the automatic nervous system can increase the activity of sympathetic and decreasing the activity of parasympathetic systems (25). Through improving the balance between sympathetic and parasympathetic systems, exercise improves the systolic function of the left ventricle, which increases cardiac output during exercise and reduces resting heart rate (26). The effect of exercise on the heart wall shows that these types of exercise increase cardiac caves, especially in the left ventricle. Left ventricle will be affected by physical activities more than other parts (27). During Pilates, cardiac output increases through simultaneous increasing of heart rate and beating volume so that tachycardia happens and diastolic time augments, less time will be given to the left ventricle for diastolic filling (28). Pitsavos et al. studied the impact of aerobics with 60% to 80% maximum consumed oxygen on the treadmill in aging women, which resulted in decreasing systolic and diastolic blood pressure after the exercise (29). In a study that examined the effects of the intensity of aerobic exercise on systolic blood pressure both in resting and during the exercise, the results showed that exercise with low and extreme intensity leads to an increase in systolic blood pressure (30). Findings of research on the study of the effects of doing exercise on arterial fibrillation arrhythmia demonstrated that exercise with extreme intensity had no superiority to reduce arterial fibrillation compared to low-intensity exercise (31). Several known mechanisms can be described for blood pressure loss due to exercise. These include reducing adrenergic tone, effects on the sympathetic system, weight loss, and body fat (32). Among other possible causes of decreasing blood pressure, reducing peripheral arterial resistance and increased cardiac output during the exercise can be mentioned (33). Reduction of blood pressure possibly happens due to the reduction of produced cathecholamine after doing exercise. This reaction is involved in decreasing peripheral resistance against circulation and consequently, reduction of blood pressure. Besides, doing an exercise can facilitate sodium secretion from kidneys and lessen the liquid volume and consequently blood pressure (34). Genetic and environmental factors may also affect the influence of sports activities on blood pressure (35). Some researchers believe after doing exercise, an enzyme called dopamine hydroxylase reduces in the hypothalamus that its reduction decreases the peripheral activity of epinephrine in response to the feelings and other stimulations and helps to lessen blood pressure (36). Longtime exercise leads to decreasing blood pressure by affecting baroreceptors. Arterial baroreceptors are located in the carotid sinus of the aortic arch. These receptors are sensitive to stretching and send blood pressure changes by nervous messages to the brain stem through changing the stretches. The messages are sent to the brain stem by baroreceptors. They make the sympathetic system active and impose entrapping effects on the number of heartbeats and therefore, blood pressure is controlled (35). Various findings have been reported about the effects of different sports activities on heart rate and resting systolic and diastolic blood pressure, which can be rooted in the diversity of studied groups, duration of doing exercise, intensity, and time. However, it is necessary to study effective factors in deeply changing these variables.