1. Background
Premenstrual syndrome (PMS) is one of the most common health problems among women. It refers to the periodic recurrence of a combination of physical, psychological, and behavioral changes during the luteal phase of the menstrual cycle (1). The association between the menstrual cycle and behavioral changes in women was first identified by Hippocrates. He considered the headaches and heaviness before menstruation are due to the turbulence in blood seeking a way to exit the body (2).
However, PMS, as a broad diagnostic concept, was first introduced in 1953 by Green and Dalton to indicate the recurrent symptoms during the premenstrual period or the first days of menstruation, which completely disappear after the cycle (1). PMS is very common among Iranian women in their menstrual periods, especially in high school students, which has reported about 80.4% in several studies. Based on the reports, 90% of the women have some of the symptoms of PMS in their cycles and nearly, 20% of the women experience behavioral and psychological symptoms of the PMS in their menstrual age (3). PMS in this vulnerable group can lead to several problems affecting the whole society (4).
Clinical symptoms of PMS can be divided into three categories: physical, mental, and behavioral, which occur periodically before menstruation and disappear when menstrual bleeding begins. Significant physical signs and symptoms of PMS include swelling, breast tenderness, headache, no appetite, and heart palpitations, and also psychological and behavioral symptoms, such as depression, irritability, fatigue, aggression, suicidal tendencies, mood swings, and social isolation (4-6).
PMS symptoms may vary from mild to moderate in women before menopause. More severe symptoms of premenstrual dysphoric disorder (PMDD) have been reported in women prior to menopause. However, the prevalence of PMDD is significantly different in various studies. Mood symptoms are more severe with harmful effects on the relationships as well as social and occupational life of the affected individuals (7). The main cause of PMS is still unclear, as it is not a single disorder and is a combination of biological factors that involve both psychological and social dimensions. Age, occupation, diet, contraceptive pills, genetic, social, and psychological factors, and no physical activity make a woman prone to PMS (8, 9).
The most important risk factors for this syndrome include hormonal imbalance, neurotransmitter disorders, hypoglycemia, hyperprolactinemia, psychosis, endorphin, vitamins and minerals, and essential fatty acid deficiency, acid-base disorders, and prostaglandin imbalance. Moreover, overweight and obesity seem to play a substantial role in some of the menstrual problems, because weight gain and especially the increased adipose tissue in the central areas of the body can result in the disruption of the steroid hormones balance, such as androgens and estrogens. Obesity may also increase the production of estrogen, which is associated with the percentage of body fat. Although numerous studies have been conducted to investigate these factors, there are few studies on the association between PMS and obesity and anthropometric indices. Moreover, no precise methods have implemented to evaluate the relevant anthropometric indices, and contradictory results have reported in this regard (8, 10, 11).
In a study on teen girls, the prevalence of 78.1% was estimated for PMS and it was severe in 13 (4.7%), moderate in 34 (12.8%) and mild in 196 (72.5%) students (9). Other variables, such as weight, body mass index (BMI), age of menarche, ethnicity, educational level, parental education, economic status, residential status, and weekly exercise were not significantly associated with PMS (12). In similar research on the association between anthropometric indices and PMS and its severity in 365 female students, a significant direct correlation was found between PMS and waist circumference (WC) as well as the waist-to-hip ratio (WHpR) (7, 11).
In an interventional study, the effect of cinnamon on menstrual bleeding and systemic symptoms of painful menstruation was assessed and the results revealed that menstrual bleeding in the cinnamon group was significantly lower than that of the control group (13).
BMI and premenstrual dysphoric disorder (PMDD) has shown to be correlated, however no correlation was found with PMS, which indicates that the higher amounts of fat are a risk factor for developing PMDD. The observed relationship of dysmenorrhea and menorrhagia with oxidative stress to both PMS and PMDD and consumption of junk food to PMDD sheds light on the possible effects of lifestyle on premenstrual disorders (14).
2. Objectives
Therefore, this study was designed to investigate the relationship between the age at menarche and PMS with anthropometric indices in high school female students of Zahedan, Iran.
3. Methods
In this cross-sectional descriptive-analytical study, 300 high school female students (15, 16) from the first and second educational districts of Zahedan were included. The samples were chosen from 10 schools located in the northern, southern, eastern, and western parts of the city. Mohammadi et al. (7) confirmed the relationship between PMS and WHpR (r = 0.125) and their reported value was used for our calculation, based on the following formula. Power of the study was set at 85% (1 - β = 0.85) and α = 0.05. Accordingly, a sample size of 284 subjects was estimated. Due to the possible attrition, a total of 300 high school female students were finally studied./
The reliability of the premenstrual symptoms screening tool (PSST), as measured by internal consistency, was satisfactory (Cronbach’s alpha coefficient = 0.93). The psychometric properties of the Iranian version of PSST were assessed by performing reliability (internal consistency) and validity (content validity ratio (CVR) and content validity index (CVI)) analyses. It’s content validity based on the CVR and CVI values was desirable (0.7 and 0.8, respectively). The Iranian version of PSST seems to be a reliable and valid measure for detecting PMS in the Iranian young women (17).
The subjects were collected using multistage sampling. In each school, the samples were selected via convenience sampling, of those willing to participate in the study.
The inclusion criterion was all healthy girls studying in Zahedan high schools willing to participate in the study. The exclusion criteria were as follows: no treatment to reduce the symptoms of PMS, unusual menstruation, mental disorders, using sedative medications, hyperthyroidism, hypothyroidism, hormone therapy drugs, cigarette and tobacco smoking, calcium and omega-3 supplementation, alcohol consumption, following a weight loss diet, stress in the last three months due to surgery, marriage and death of relatives, suffering from specific chronic diseases (such as diabetes, hypo- and hyperthyroidism, and hypertension), and unwillingness to participate in the study. The questionnaires were distributed among the students after making the required coordination with the authorities of the selected schools, and making the subjects informed about the goals and terms of the study and also the confidentiality of information.
The questionnaire was randomly filled out by 20 female students residing in dormitories (pilot study), before it was reviewed and evaluated according to the participants’ responses. In the next step, it was developed in three sections, including general characteristics of the participants, anthropometric indices (i.e. weight and height, WC, and WHpR, and the level of PMS.
Demographic characteristics, including age and marital status were recorded. The studied anthropometric indices were weight, height, WC and WHpR. Weight (kilogram) was measured with an accuracy of 0.1 kg with minimal clothing, whereas the height of participants was determined using a vertical non-flexible measuring tape for each person with an accuracy of 0.5 cm without shoes. Similarly, WC (the shortest circumference of the area between the lowest rib and the iliac crest) and WHpR (the highest circumference of the pelvic bone) were measured in centimeters with an accuracy of 0.5 using a non-elastic measuring tape with a minimal level of body covering. BMI and WHpR were calculated, as well.
The PMS symptoms questionnaire was used to assess PMS and premenstrual symptoms. This self-report scale includes 21 questions that measure the frequency and severity of PMS symptoms, of which 10 questions assess psychological symptoms, 10 questions measure physical symptoms, and one question is related to the degree of adjustment disorder. Its content was derived from the DSM-IV diagnostic criteria for PMDD and PMS symptoms. The respondents were asked to mark the severity of symptoms on a 5-point Likert scale (none = 0, mild = 1, moderate = 2, relatively high = 3, and severe = 4). In order to determine the prevalence of PMS, according to the guidelines developed by the American College of Obstetricians and Gynecologists for PMS, the subjects with at least 2 symptoms listed in the PMS scale (at least one emotional and one physical symptom) with moderate to above moderate severity were identified with PMS. It should also be mentioned that the validity and reliability of this questionnaire have been previously confirmed (17, 18).
According to the standardized PMS questionnaire, a person had to meet the following requirements to be regarded as having moderate to severe PMS:
A) From the first 4 questions, at least one response should be scored as moderate or severe.
B) From the first 14 questions, at least 4 responses should be scored as moderate or severe.
C) From the last 5 questions, at least one response should be scored as moderate or severe.
Other affected subjects were placed in the mild PMS group.
In order to collect the information via the questionnaire or in-person interview, the required arrangements were made with the authorities of each educational district, school, and the teachers and, if necessary, a health care instructor or an educational counselor.
3.1. Statistical Analysis
SPSS version 22 was used to analyze the data through descriptive statistics (mean and standard deviation) and also the differences for each quantitative variable. To analyze the quantitative data, a comparison was made between the mean of the two groups using the independent t-test. The chi-square test was used to compare the qualitative data between the groups. In order to study the correlation between PMS intensity and quantitative variables, Pearson’s correlation test was employed. A value of P <0.05 was considered to suggest a significant difference or relationship between data.
The participants were informed about the research objective and their consent was obtained before the study. Those who did not meet the inclusion criteria were excluded and replaced by other subjects. This research was approved (approval code: IR.ZAUMS.REC1396.170) by the Ethics Committee and the Vice Chancellor for Research and Information Technology of Zahedan University of Medical Sciences.
4. Results
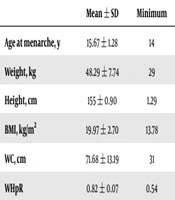
In this study, 300 high school female students with the mean age of 15.7 ± 1.3 years (range, 14 - 20 years) were studies. The subjects’ mean age at menarche was 15.67 ± 1.28 years and their mean weight and height were 48.29 ± 7.74 kg and 155 ± 0.90 cm, respectively. The mean BMI, WC, and WHpR were also 19.97 ± 2.70 kg/m2, 71.68 ± 13.19 cm, and 0.82 ± 0.07, respectively (Table 1).
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| Age at menarche, y | 15.67 ± 1.28 | 14 | 20 |
| Weight, kg | 48.29 ± 7.74 | 29 | 80 |
| Height, cm | 155 ± 0.90 | 1.29 | 1.75 |
| BMI, kg/m2 | 19.97 ± 2.70 | 13.78 | 30.86 |
| WC, cm | 71.68 ± 13.19 | 31 | 105 |
| WHpR | 0.82 ± 0.07 | 0.54 | 0.98 |
Abbreviation: BMI, body mass index.
The highest BMI was seen in the 17-year-old age group (20.55 ± 3.28 kg/m2), whereas the minimum BMI was related to the 16-year-old age group (19.51 ± 2.60 kg/m2).
Based on the results, 221 subjects (73.7%) had experienced PMS and 79 subjects (26.3%) had no syndrome. According to the results, the highest PMS score was seen in the 19-year-old age group (9.60 ± 6.42), whereas the lowest PMS score was found in the age group of 18 years (5.09 ± 4.15).
Based on Pearson’s correlation test results, there was no significant relationship between the mean age at menarche and BMI and also between the mean age of menarche and WC and WHpR. However, there was a significant positive correlation between the mean age at menarche (P = 0.004) and height (P = 0.025) in the students.
As indicated by the independent t-test, there was no significant relationship between PMS prevalence and anthropometric indices in the high school female students (Table 2).
| Values | P Value | |
|---|---|---|
| Age at menarche, y | 0.214 | |
| Negative | 15.51 ± 1.35 | |
| Positive | 15.72 ± 1.25 | |
| BMI, kg/m2 | 0.073 | |
| Negative | 19.50 ± 2.50 | |
| Positive | 20.14 ± 2.75 | |
| WC, cm | 0.559 | |
| Negative | 72.35 ± 13.38 | |
| Positive | 71.44 ± 13.15 | |
| WHpR | 0.658 | |
| Negative | 0.83 ± 0.07 | |
| Positive | 0.82 ± 0.07 |
Abbreviation: BMI, body mass index.
aNegative number = 79, positive number = 221.
bValues are expressed as mean ± SD.
Based on the results of independent t-test, moderate and severe types of PMS (in terms of emotional symptoms) did not have a significant relationship with the age of menarche (P = 0.77), the anthropometric indices of BMI (P = 0.45), WC (P = 0.74), and WHpR (P = 0.79) (Table 3).
| Values | P Value | |
|---|---|---|
| Age at menarche, y | 0.766 | |
| Mild | 15.64 ± 1.29 | |
| Severe | 15.68 ± 1.28 | |
| BMI, kg/m2 | 0454 | |
| Mild | 19.82 ± 2.57 | |
| Severe | 20.06 ± 2.78 | |
| WC, cm | 0.746 | |
| Mild | 72.00 ± 13.18 | |
| Severe | 71.49 ± 13.23 | |
| WHpR | 0.789 | |
| Mild | 0.82 ± 0.08 | |
| Severe | 0.82 ± 0.07 |
Abbreviation: BMI, body mass index.
aMild number = 110, Severe number = 190.
bValues are expressed as mean ± SD.
The results of independent t-test showed a significant relationship between the mild and severe PMS (in terms of physical symptoms) and the age of menarche (P = 0.038). Other anthropometric indices, including BMI (P = 0.22), WC (P = 0.24), and WHpR (P = 0.11) showed no significant difference in the subjects (Table 4).
| Values | P Value | |
|---|---|---|
| Age at menarche, y | 0.038 | |
| Mild | 15.52 ± 1.31 | |
| Severe | 15.82 ± 1.23 | |
| BMI, kg/m2 | 0.227 | |
| Mild | 19.79 ± 2.53 | |
| Severe | 20.17 ± 2.86 | |
| WC, cm | 0.244 | |
| Mild | 72.55 ± 12.94 | |
| Severe | 70.77 ± 13.44 | |
| WHpR | 0.116 | |
| Mild | 0.83 ± 0.07 | |
| Severe | 0.82 ± 0.07 |
Abbreviation: BMI, body mass index.
aMild number = 153, Severe number = 147.
bValues are expressed as mean ± SD.
Furthermore, the status of physical factors and adaptation to the mild and severe stages of PMS were not significantly correlated with the age of menarche and other anthropometric indices (P > 0.05; Table 5).
| Values | P Value | |
|---|---|---|
| Age at menarche (year) | 0.655 | |
| Mild | 15.63 ± 1.34 | |
| Severe | 15.70 ± 1.22 | |
| BMI (kg/m 2) | 0.415 | |
| Mild | 19.84 ± 2.63 | |
| Severe | 20.10 ± 2.76 | |
| WC (cm) | 0.990 | |
| Mild | 71.67 ± 13.43 | |
| Severe | 71.69 ± 13.00 | |
| WHpR | 0.648 | |
| Mild | 0.82 ± 0.07 | |
| Severe | 0.82 ± 0.07 |
Abbreviation: BMI, body mass index.
aMild number = 147, Severe number = 153.
bValues are expressed as mean ± SD.
5. Discussion
According to the findings, the mean age of menarche was 15.67 ± 1.28 years (range, 14 - 20) in high school female students of Zahedan. In a study by Ramezanpour et al. in Gonabad, east of Iran (10), the mean age at menarche was 13.44 ± 1.16 years, whereas it was reported 13.36 ± 1.34 years in Bushehr, southwest Iran (19). The age at menarche is influenced by different factors, such as genetic, economic, social, and health-related factors as well as body fat, diet, climate, geographic location, and lifestyle. The higher mean age at the menarche in our research can be due to the climatic, geographical, and regional features (20); however, further studies are needed to confirm this. High school female students need to be supported by the family and community at this age in order to overcome PMS, because it is usually associated with physiological problems. These girls are emotionally vulnerable, and their exposure to this phenomenon can lead to more emotional disorders.
Paying more attention to the nutritional status of the subjects can improve their physical and physiological health, making them prepare to deal with health problems, such as PMS. However, lifestyle and cultural differences can also be effective against PMS (21).
In the present study, the mean WC of the participants in the healthy students and those with PMS was 72.35 ± 13.38 and 71.44 ± 13.15 cm, respectively. The mean WHpR in the healthy students and those with PMS was also 86.60 ± 10.97 and 85.99 ± 11.05, respectively. Moreover, the mean WHpR in healthy students was 0.83 ± 0.07 and 0.82 ± 0.07 in students with PMS. Both WC and WHpR were within the acceptable range in both PMS and healthy subjects and no significant difference was observed; however, WHpR showed a slight increase compared to the standard level. In the study by Jafarirad et al. on students in Ahwaz (22), WC and WHpR were 72.4 ± 7.05 cm and 0.74 ± 0.05 in healthy students, respectively. Comparing these values demonstrated that healthy girls had more favorable anthropometric indices than girls with PMS. Therefore, it is suggested to improve the nutritional status and physical health of people with PMS (21). Based on the results of the PMS scale, 221 subjects (73.7%) had PMS and 79 subjects (26.3%) were had no PMS. Although this result is consistent with some other studies in Iran and worldwide, this is not consistent with several other reports. The widespread prevalence of PMS has reported in Iranian menstruating women, especially among high school students (4).
The prevalence of PMS was 35.6% in Saudi Arabia (23), 51% in Pakistan (24), and 78.1% in Brazil (25). In general, comparing the estimates of PMS prevalence in the present study with those of Iranian and global studies indicates several similarities and differences. The existing dissimilarities may arise from using different tools for evaluating PMS, different sample sizes and social characteristics of the subjects, and also distinct inclusion and exclusion criteria used in each investigation (10).
There was no significant difference in anthropometric indices in the studied population in terms of PMS level. However, this difference was significant in terms of the age at menarche for subjects with mild and severe PMS. Hence, more attention should be paid to the nutritional status of adolescents before menarche, by which they can be physically prepared to overcome PMS complications. Body reserves, including fats, are helpful to prepare the conditions for a proper and timely experience of the Biological age in young girls (10, 12).
There was a significant relationship between the mean age at menarche and weight and height, and also between the scores of physical symptoms of PMS and the age at menarche in high school girls. Therefore, not only considering nutritional and health-related aspects of life, but also focusing on other dimensions of lifestyle, such as physical activity, and mental health during this high risk period can be an important and effective way to deal with PMS in this vulnerable population (7, 26).
5.1. Conclusions
The results indicated great variations in the range of menopausal age, which implies the participants’ vulnerability. A significant relationship was identified between the weight and height indices of the adolescent girls and also the physical factors of PMS and the age at menarche, in high school female students. Improving nutritional habits, promoting anthropometric indices, and evaluating more PMS-related indicators, mental health problems, the age at menarche, hormonal balance, and other social and cultural factors, and also conducting supportive nutritional interventions coupled with usual educational programs as well as raising public awareness can be effective in mitigating PMS in adolescent girls. It is strongly recommended that extensive studies be conducted with larger sample sizes and intensive monitoring in order to achieve more conclusive results.