1. Background
Breast cancer is a major cause of malignancy and death in women (1). Mammary cancer is common in older intact female dogs (2). The murine 4T1 mammary carcinoma is one of the best breast cancer models because of its capacity to metastasize efficiently to various distant tissues such as lymph nodes, brain, blood, bone, and respiratory system (3).
The immune surveillance theory declares that immunity can recognize and delay tumor development, at least in the early stage of malignancy (4). Nevertheless, some cancer cells can evade the pressure of the immune response, thereby developing a malignancy. Therefore, the enhancement of immune responses against malignant cells may be a logical approach to control tumors (4-6). The polarization of immune cells towards cellular responses has a pivotal role in defense against malignancy (7). Adjuvants are pharmacological and/or immunological agents employed for the induction of an appropriate response in clinical vaccines (8, 9). Inorganic aluminum salts (alum) are unique adjuvants allowed by the United States Food and Drug Administration (FDA). However, alum has some limitations. The fundamental shortcoming of aluminum salts is their incapacity to promote cytotoxic T cells or T helper1 (Th1) responses that are needed to eradicate most tumor cells (9, 10). Thus, it is logical to carry out further research on safe and new adjuvants to promote a profound cellular immunity.
The sympathetic nervous system innervates all lymph nodes and spleen, and the end products of this system (like norepinephrine and epinephrine) modulate various arms of immunity (10, 11). It has been documented that β-adrenergic antagonists can elicit both cellular and humoral immune responses against Salmonella typhimurium (12) and Plasmodium berghei (13). Moreover, it has been reported that stress-related hormones (like epinephrine and norepinephrine) have a pivotal role in the initiation and progression of tumors (14).
2. Objectives
Accordingly, the current survey was designed to evaluate the beneficial effects of a new immunotherapy method against the animal model of breast malignancy induced by combined heated 4T1 cells as a source of tumor antigens, and propranolol, as an adjuvant.
3. Methods
3.1. Reagents
Dulbecco’s modified eagle medium (DMEM) and fetal calf serum (FCS) were obtained from GIBCO/Life Technologies Inc. (Gaithersburg, MD). The Griess reagent kit was procured from Cayman Chemical (USA). The lactate dehydrogenase (LDH) cytotoxicity detection kit was obtained from the Takara Company (Tehran, Iran). The ELISA kits were obtained from Qiagen (Hilden, Germany). Other reagents were procured from Sigma-Aldrich (St. Louis, MO).
3.2. Cells and Culture Conditions
The 4T1 mammary carcinoma cells were provided by the Iran Pasteur Institute. The cells were passaged at the 37°C humidified atmosphere with 5% CO2 in a standard culture medium (DMEM supplemented with 10% FBS).
3.3. Animals and Tumor Induction
Female BALB/c mice (six to eight-weeks-old) were obtained from the Pasteur Institute of Iran and maintained under the constant condition of temperature (22°C - 24°C) with a dark/light cycle of 12/12 h. Animals were given water and food ad libitum. They were subcutaneously implanted in the right flanks with 1 × 104 viable 4T1 cells in 50 µL of phosphate-buffered saline (PBS). The growth rate of the tumor was checked every four days by a caliper. The volume of the tumor (mm3) was computed using the formula of an ellipsoid (width × height × length × 0.5236) (14, 15). Ethical issues were met as per the orders of the Ministry of Health, the I.R. Iran, approved by the Medical Ethics Committee of the Veterinary University.
3.4. Immunization of Mice
Immunization began when all mice had shown a palpable tumor. Afterward, the animals were randomly allocated into three equal groups (n = 10). All immunizations were done by injecting through the subcutaneous (S.C.) route. In the first group, tumor control animals, mice were twice treated with 100 µL of PBS in one week. In the second population, heated 4T1-treated group, animals received twice, at a one-week interval, the freeze and thaw extract of 106 heated 4T1 cells in 100 µL volumes. Heated 4T1 cells were prepared by using the disposal of 4T1 cells to non-lethal heat shock. In brief, 4T1 cells at 85% confluence were warmed for 30 min in a 42°C water bath, followed by recovering for 2 h at 37°C. Then, the tumor cells were frozen in a liquid nitrogen tank for 10 min and thawed three times at 4°C. The extract was centrifuged at 15,000 g for 20 min. The isolated supernatant was maintained at -80°C as tumor cell lysate (15). Finally, in the last group, combined propranolol and heated 4T1-treated animals, mice were twice immunized at a one-week interval with the freeze and thaw extract of 106 heated 4T1cells and propranolol (6 mg/kg) in 100 µL volumes. This dose was chosen as per the previous work on the adjuvant properties of propranolol (12, 13). Seven days after the ultimate immunotherapy, half of the animals were sacrificed to monitor the cytokine production in splenocytes. Other mice were monitored for the comparison of the survival curve and the mammary tumor size.
3.5. Splenocytes Proliferation and Cytokine Production
Splenocytes were aseptically detached from each mouse. After the preparation of a single-cell suspension, erythrocytes were omitted by Gey’s solution. Cells (2 × 106/mL) were kept in 24-well plates and pulsed with antigens derived from tumor cells by freeze and thaw (100 µg/mL) and 25 µL of phytohemagglutinin solution (1 mg/mL). Tumor antigen was prepared as described earlier (16). The culture supernatants were removed after 72 h. The TGF-β, IFN-γ, and IL-10 products were checked by the sandwich ELISA method according to the manufacturer’s instructions.
The proliferation potential of lymphocytes in splenocytes was evaluated by the MTT reduction assay method. Splenocytes were plated in 96-well flat-bottomed plates (1 × 105 cells/100 µL/well) and pulsed with antigens derived from tumor cells by freeze and thaw (100 µg/mL) and 25 µL of phytohemagglutinin solution (1 mg/mL) or the medium alone. After 72 h of incubation, 20 µL of the MTT solution (5 mg/mL) was added to each well and cultured at 37°C for 4 h. Then, dimethyl sulfoxide (150 µL) was added to each well and shaken strongly to solve formazan crystals. The optical density was monitored by a standard microplate reader at 550 nm (Dynatech, Denkendorf, Germany). The tests were repeated three times. Data were finally reported as the proliferation index according to the ratio of OD550 of pulsed wells with phytohemagglutinin to OD550 of non-pulsed wells (16).
3.6. NK Cytotoxicity Assay
The NK cytotoxicity was evaluated by the LDH assay. In this assay, the live 4T1 and splenocytes, as a source of NK cells, were used as target and effector cells, respectively. The assay was done as described previously. In brief, both cells were twice rinsed with the assay medium (RPMI1640 with 1% bovine serum albumin) and co-cultured in 96-well flat-bottomed plates for 6 h at 37°C at a ratio of 50 effector cells to one target cell in 200 µL of culture medium. Then, 100 µL of the LDH detection admixture was added to each well. The mixture was cultured for 25 min at room temperature. The optical density was recorded by using a microplate reader at 490 nm. The percentage of cell-mediated cytotoxicity was calculated by the below formula:
Cytotoxicity% = (experimental release - spontaneous target release - spontaneous effector release)/(maximal target release–spontaneous target release) × 100
3.7. Potential of Respiratory Burst in Splenocytes
The potential of reactive oxygen species production in the population of splenocytes was evaluated by using the nitroblue tetrazolium (NBT) dye reduction exam as described earlier (17). Briefly, 0.1 ml of S. aureus suspension (108 cell/mL) was mixed with 0.1 mL of 0.1% NBT solution and 0.1 mL of the suspension of splenocytes and incubated for 20 min at 37°C. Reduced NBT was drown out in dioxane and measured at 520 nm.
3.8. Assessment of Nitric Oxide in Splenocyte Population
The potential of nitric oxide production by splenocytes was evaluated using the Griess reagent (18). After the splenocytes culture, 50 µL of the supernatant was collected and combined with 50 µL of Griess reagent (3% phosphoric acid, 0.1% naphthyl ethylenediamine, and 0.1% sulfanilamide). The mixture was kept in the dark for 15 min. Then, optical absorbance was checked at 540 nm by using a standard microplate reader (Dynatech, Denkendorf, Germany). The concentration of nitrite was finally monitored based on a standard curve.
3.9. Statistical Analysis
Data were analyzed using MedCalc Software (version 19.1.3, MedCalc Software, Mariakerke, Belgium). The Kaplan-Meier estimator was applied to check the survivability of tumor-bearing mice. Repeated-measures analysis of variance (ANOVA) was used to monitor the mammary tumor size. Other data were analyzed using the Kruskal-Wallis one-way analysis of variance, followed by pair-wise comparisons using the Wilcoxon rank-sum test with Bonferroni correction. The Kaplan-Meier estimator was applied to check the survivability of tumor-bearing mice. The data were reported as mean ± SD. Values of P < 0.05 were considered statistically significant.
4. Results
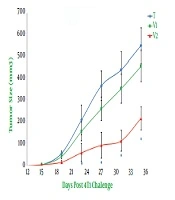
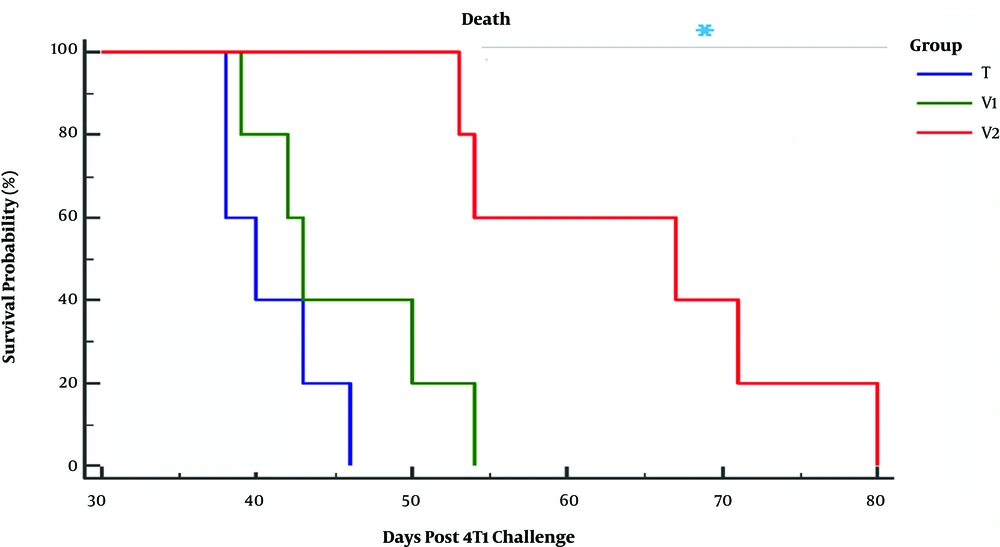
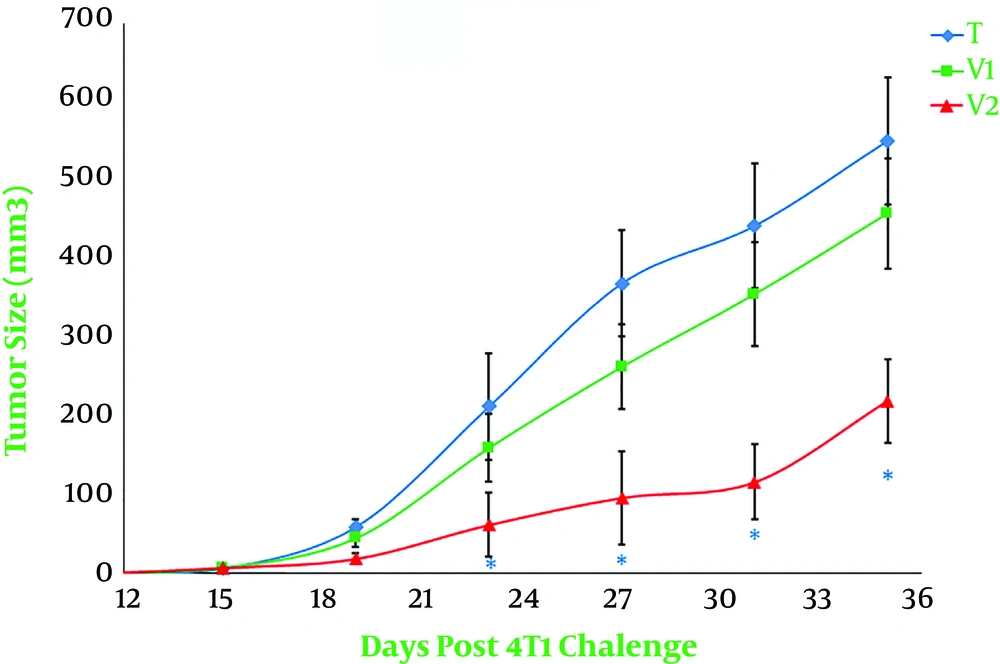
The animals were checked every five days following the injection of live 4T1 cells for the first signs of a tangible tumor that continued until day 80 when all the animals died. The vaccination was initiated on day 11 after the 4T1 injection when all mice brought up a palpable tumor. The evaluation of the survival curve by using the Kaplan-Meier estimator indicated that tumor-bearing mice receiving combined immunotherapy showed a significant reduction in the mortality rate when compared to other tumor-bearing mice (P < 0.001, Figure 1). Statistical analysis also demonstrated no significant difference in the survival rate between tumor-bearing mice vaccinated with propranolol or extract of heated 4T1 cells (P = 0.25, Figure 1). At least 20% of the tumor-bearing mice vaccinated with the combination of propranolol and extract of heated 4T1 cells were alive until day 79 after live 4T1 injection, while all tumor-bearing animals in the group treated with an extract of heated tumor died 54 days after treatment with live 4T1 cells (Figure 1). The untreated mice showed a very poor viability rate, so that all of the tumor-bearing animals in this group died on day 44 after live 4T1 injection (Figure 1). As exhibited in Figure 2, tumor growth also showed a significantly lower rate of development in tumor-bearing mice receiving combined immunotherapy than in the other groups. The regression of the rate of tumor growth was initiated in this group from day 24 after tumor induction. The value of tumor volume was not statistically different between the animals receiving only the extract of heated 4T1 cells and tumor-bearing animals in the control group (Figure 2).
Comparison of survival curve of 4T1 challenged Balb/c mice. Immunotherapy was performed when all of the animals had a palpable tumor. T, Tumor-bearing mice; V1 and V2, tumor-bearing mice twice immunized at a one-week interval with the extract of heated 4T1 (1 × 105) alone and in combination with propranolol (3 mg/kg), respectively.
Monitoring the mammary tumor size. The tumor size was estimated every five days using a digital caliper. Tumor volume (mm3) was measured as detailed in materials and methods. T, Tumor-bearing mice; V1 and V2, tumor-bearing mice twice immunized at a one-week interval with the extract of heated 4T1 (1 × 105) alone and in combination with propranolol (3 mg/kg), respectively. (*, P < 0.01 versus other groups).
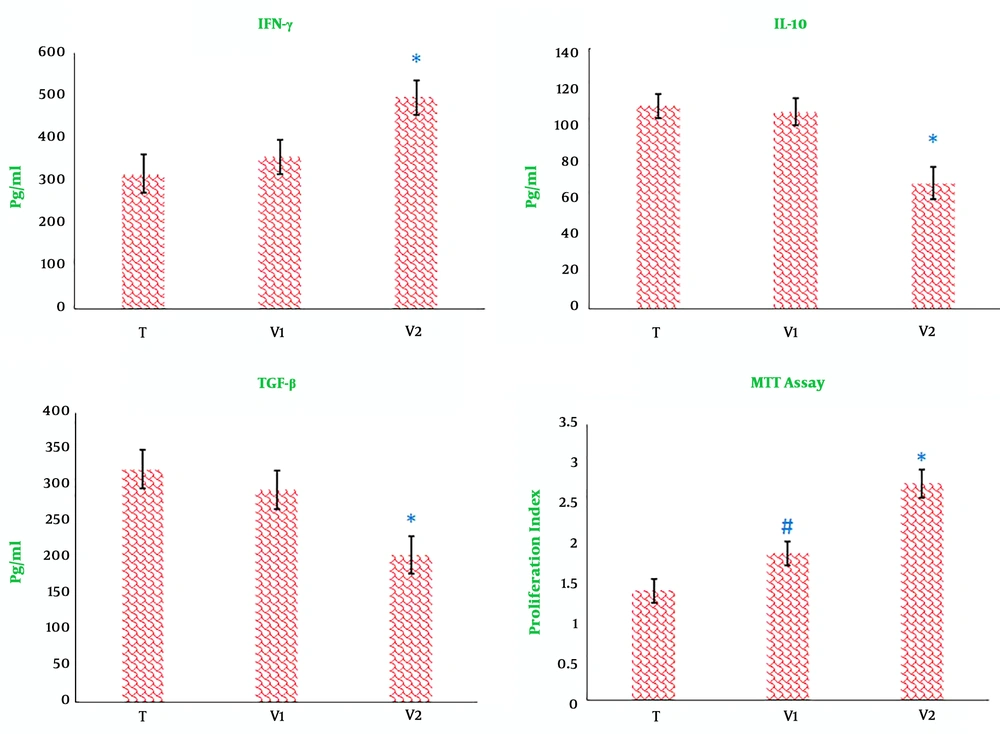
The ex vivo cytokine monitoring showed that the extract of heated 4T1 cells combined with propranolol significantly promoted the profound production of IFN-γ and, conversely, down-regulated the production of TGF-β and IL-10 in the splenocytes, when compared to the splenocyte population from tumor-bearing controls and tumor-bearing mice treated with the extract of heated 4T1 cells alone (Figure 3). The monitoring of splenocyte proliferation showed a significant increase in mice with tumors that were treated with combined propranolol and heated 4T1 cells or the extract of heated 4T1 cells alone. However, combined immunotherapy with heated 4T1 cells and propranolol caused a more prominent increase in the proliferation index when compared to tumor-bearing mice treated with the extract of heated 4T1 cells alone (Figure 3).
Effects of immunotherapy on cytokine production by splenocytes. Half of the mice in each group were sacrificed 10 days after the last immunotherapy, and splenocytes were cultured for 72 h under conditions described in materials and methods. T, Tumor-bearing mice; V1 and V2, tumor-bearing mice twice immunized at a one-week interval with the extract of heated 4T1 (1 × 105) alone and in combination with propranolol (3 mg/kg), respectively. (*, P < 0.01 versus other tumor-bearing mice; #, P < 0. 01 versus tumor-bearing mice receiving DCs maturated heated candida extract or control tumor-bearing mice).
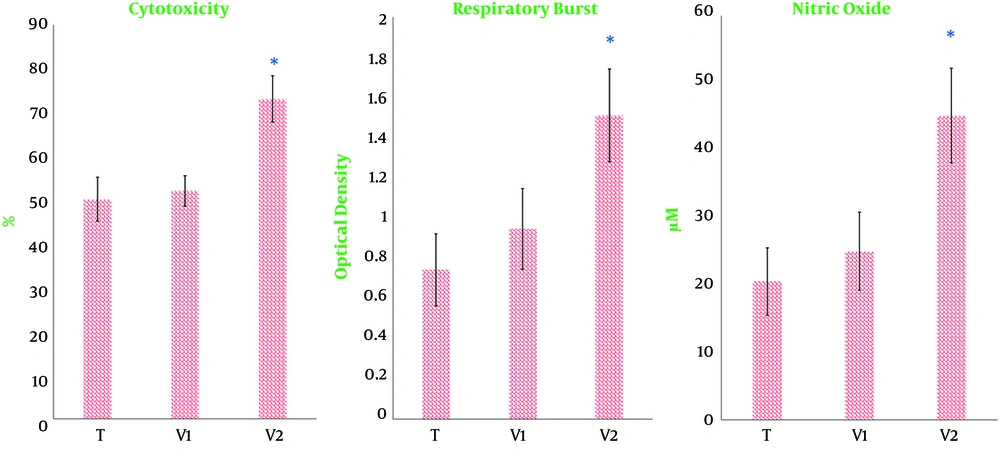
The effects of immunotherapy on natural killer cell-mediated cytotoxicity were determined by measuring the levels of LDH released from 4T1 cells after challenging with natural killer cells in the splenocyte population. The gained data indicated that natural killer cell cytotoxicity significantly increased only in tumor-bearing mice treated with combined immunotherapy (Figure 4). As shown in Figure 4, respiratory burst and nitric oxide production by splenocytes were significantly up-regulated in the splenocytes from tumor-bearing mice treated with combined immunotherapy and those receiving only heated tumor cells when compared to tumor-bearing controls. Nonetheless, this increase was significantly much more eminent in tumor-bearing mice receiving combined immunotherapy than in tumor-bearing mice treated only with heated tumor cells (Figure 4).
Effect of immunotherapy on respiratory burst (A) and nitric oxide production (B) in the splenocyte population. Half of the mice in each group were euthanized 10 days after the last vaccination, and splenocytes were isolated and cultured for 72 h under conditions described in materials and methods. T, tumor-bearing mice; V1 and V2, Tumor-bearing mice twice immunized at a one-week interval with the extract of heated 4T1 (1 × 105) alone or in combination with propranolol (3 mg/kg), respectively. (*, P < 0.001 versus other tumor-bearing mice; #, P < 0. 01 versus tumor-bearing mice receiving DCs maturated heated candida extract or control tumor-bearing mice); #, the combination of propranolol and heated 4T1.
5. Discussion
The purpose of the current investigation was to evaluate the efficacy of a combination of the extract of heated 4T1 cells and propranolol for the improvement of murine 4T1 mammary carcinoma in BALB/c mice. It is worth noting that the protocol of immunotherapy should have a good safety margin. Therefore, in the current study, we used propranolol that is frequently prescribed in humans. Despite the useful results, no notable adverse event was observed in this study. Moreover, attained data indicated that the combined immunotherapy with propranolol and the extract of heated 4T1 cells had synergistic effects, causing a more desirable survival curve and slower malignancy growth when compared to other tumor-bearing mice that received only heated 4T1 or PBS. Concurrent with these results, low cost and appropriate safety margin (19) introduced propranolol as an ideal candidate for combination with tumor antigens. Similar to our results, it has been shown that the tumor lysate of fibrosarcoma and propranolol could reduce tumor growth (20).
Sympathomimetic agents released from adrenal glands or sympathetic nerves, and interestingly from antigen presenting cells (APCs), could modulate immune responses (13). It is clear that epinephrine and norepinephrine, especially via the stimulation of β-adrenergic receptors, can limit Th1 immune responses and direct immunity toward the Th2 profile (21, 22). Therefore, beta-blockers like propranolol may promote immunity towards Th1, an essential anti-tumor arm of immunity. Similar to the current work, several reports also indicated that beta-blockers can potentiate immune responses (12, 13, 21). Nadolol, a beta-receptor antagonist, can reportedly augment cytotoxic T cells against influenza A virus infection (23). Antigen-presenting Cells (APCs) have β-adrenergic receptors (24). Propranolol can block these receptors and promote local inflammation by reducing the local effects of antigen-presenting cells (APC)-, adrenal gland, or sympathetic nerve-secreted catecholamines (24, 25). The suppression of regulatory T lymphocytes (Tregs) is another possible mechanism to accelerate cell-mediated immunity by propranolol (20, 26). Treg cells are major sources of IL-10 and TGF-β, two potent immunosuppressive cytokines (26). Interestingly, our results demonstrated that the combined immunotherapy significantly decreased the production of IL-10 and TGF-β in splenocytes compared to the production of cytokines from splenocytes in other groups. Tumor cells also escape immunity by the secretion of TGF-β and IL-10 (27). As known, IL-10 and TGF-β can regress the proliferation of lymphocytes and activation of M1 anti-tumor macrophages (28). The data also revealed that the use of propranolol, as an adjuvant in combination with the extract of heated 4T1 cells, significantly augmented the production of IFN-γ and nitric oxide, as well as respiratory burst. As is clear, IFN-γ possesses potent anti-tumor activity (14). Nowadays, it is accepted that macrophages have remarkable plastic nature. They may show a reprogramming based on their milieus (17). Besides, M1 anti-tumor macrophages can produce many cytotoxic factors for tumors like reactive nitrogen and oxygen species (17). However, cancer can induce anti-tumor macrophages with potency to promote tumor growth via the production of trophic and growth factors with very low production of reactive nitrogen and oxygen species (27, 28). Considering the published documents, the adrenergic system can impress not only the malignancy progress directly but also the functions of macrophages (14, 29). As is clear, β2 adrenergic receptor promotes breast cancer progression through the induction of M2 macrophages (14). The data in this study exhibited that the production of nitric oxide and reactive oxygen species were up-regulated in cancer-bearing mice receiving the combined immunotherapy more than in tumor-bearing mice receiving the extract of heated 4T1. Previously, the regression of nitric oxide production by macrophages via the activation of β adrenergic receptors has been documented (30).
In addition to macrophages, natural killer (NK) cells are also essential innate cells involved in the elimination of malignant cells. These cells eliminate tumor cells directly by the initiation of apoptosis in tumor cells and the secretion of cytokines like IFN-γ (31). Lactate Dehydrogenase (LDH) cytotoxicity is a simple and rapid experiment to quantify cytotoxicity by determining LDH activity released from injured cells (27). Our data suggested that cytotoxicity of NK cells was significantly elevated only in cancer-bearing mice treated with the extract of heated 4T1 cells and propranolol.
In this study, we used the extract of heated 4T1 cells as the source of tumor antigens. The induction of heat shock proteins (HSPs) by heat shock is a logical approach to promote cell-mediated immunity against tumors (32). The HSPs, especially Hsp70, are molecular chaperone families binding to tumor antigens that potentiate their phagocytosis into antigen APCs (33). The HSP-antigen complexes can initiate the cross-presentation of antigens by APCs and therefore, promote the generation of cytotoxic T cells against tumor antigens (33-35). In this survey, a sublethal heated dose was applied before 4T1 cells underwent the freeze-and-thaw procedure. In this regard, it has been previously reported that a nonlethal heat shock can reduce the growth rate and metastatic potential of 4T1 cells in vivo via Hsp70 induction (36).
In conclusion, ameliorating the tumor growth rate and better survivability in tumor-bearing mice receiving the extract of heated tumor cells combined with propranolol indicated that this approach is a favored strategy. It can be noted that breast cancer induced by 4T1 cells has a low-immunogenic property (27, 37). This is only a preliminary study, and further in vivo investigations should be designed to draw a clear conclusion, especially with a larger group of animals.