1. Background
According to estimates, cancer results in more deaths than all heart diseases and wholly stroke over the recent decade (1). Cancer cells can be characterized as altered cells by unregulated cell growth and metastasis to other parts of the body (2). Despite valuable progress in cancer therapy, various patients have a less than perfect reaction (3). Therefore, the central desire in the field of cancer treatment is the use of metals for new anticancer drugs with the potential of being harmless and exhibiting more antiproliferative activity against tumors. Recently, nanoparticles (NPs) are emerging as a novel beneficial agent for cancer therapy in a way that many trainings have been developed to study the influence of NPs in cell culture (4, 5). Copper oxide NPs (CuO NPs) can remarkably decrease cell viability in cancer cells (6-10). Various methods can be used to synthesize CuO NPs, such as sol-gel (11), ultrasonic mixing and self-assembly methods (12), microwave irradiation (13), precipitation (14), electrochemical, and so on (15). To the best of our knowledge, all of the mentioned processes are harmful materials for synthesizing CuO NPs except the green synthesis of CuO from natural resources in a way that it has emerged as a convenient, non-toxic, mild, and rapid process (16). Green production of NPs using plant extract is a new achievement for improving the elimination of elaborate processes as well as meeting large-scale production (17). Green synthesis of CuO NPs has various advantages, among which simplicity and eco-friendly are the most important features. In order to do this, CuO NPs can be synthesized from plants, which creates a more stable particle. Moreover, this process leads to the production of NPs in different sizes and shapes in comparison to the other methods. Also, it is inexpensive and a faster process (18). Recently, attention to natural products for the treatment of malignancies has been increased in a scientific population (19). Aloe vera is a succulent plant species and has been frequently used as a natural therapy for the treatment of diverse exterior illnesses (20). Anthraquinones of Aloe vera plants such as anthranol, Aloetic acid, aloin, barbaloin, emodin, chrysophanic acid, cinnamic acid, resistanol possess mutagenic and genotoxic special effects in mammalian and bacterial test systems (21). In addition, it has been found that extracts of Aloe vera exhibit cytotoxicity besides human malignancy cell lines. Even the anti‐mutagenic and anti‐leukemic special effects of isolated compounds from Aloe vera Linne have been demonstrated in-vitro (22). One suitable approach to control cancer is identifying a rational mixture of new medications or surviving anticancer agents.
Cell culture is the first step to recognize, assess, and prioritize chemical agents and natural products with the aim of anticancer effects (23). Human leukemic cell line K562 is a kind of non-adherent cell and was proven from the pleural outpouring of a 53-year-old female through chronic myelogenous leukaemia (CML) in blast crisis (24). In some studies, anticancer effects of Aloe vera have been documented (25). On the other hand, nanotechnology is one of the best rapidly developing industries, especially in biomedicine (26).
The toxicity of nanoparticles (NPs) such as copper oxide (CuO) NPs in yeast, crustaceans, protozoan, bacteria, and microalgae has been revealed (27). Moreover, there is some evidence reporting the harmfulness of CuO NPs in some cancer cell lines (28). Multidrug techniques have been used to take care of such sophisticated syndromes as tumors, hypertension, and diabetes. Consequently, in various cases, cancer cannot be removed by a remedy that objectives a single pathway, necessary protein, or gene. To gain better antitumor effects, adjusted mixtures of multiple drugs tend to be practical to synergistically assault different pathways. Combination therapy by selective therapeutic agents potentially regresses drug resistance while simultaneously promote anticancer benefits. However, the consequences of exposure to combined walnut shell-extracted CuO NPs and extracts of Aloe vera in K562-Lucena have not yet been studied.
2. Objecives
In this study, we tried to know whether combining hydroalcoholic extract of Aloe vera and green CuO NPs produced by an eco-friendly method and non-toxic walnut shells could provide synergistic cytotoxicity against K562 cell line and peripheral blood mononuclear cells (PBMC) as a normal cell.
3. Methods
3.1. Ethical Considerations
All experimental procedures were performed in accordance with the guidelines Ethics Committee of Urmia University (code Number: 707/TDT/7). The blood samples were given after approval by the institutional Health Research Ethics Specialist. More importantly, an informed consent form was signed by donors.
3.2. Chemicals
This experimental study was granted by institutional ethical permission and was fully sponsored by Urmia University. The study was done from 01/01/2018 to 22/04/2018. All Chemicals except CuO NPs (60 - 80 nm particle size cat no: 774103) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All the culture media (Fetal bovine serum (FBS) and RPMI medium) were supplied from GIBCO/Life Technologies Inc. (Gaithersburg, MD, USA). Moreover, 3- [4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT), penicillin, streptomycin, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich.
3.3. Green Synthesis of CuO NPs
To reach this goal, we used the coprecipitation method to coat the nanoparticles on the walnut shell (29). The walnut shells (WS) used in this work were obtained from a local walnut tree in Urmia, Iran. Walnut shells were washed, dried at room temperature, relented using a high-speed rotary cutting mill and then, and sieved to collect the particles with a size smaller than 0.45 mm. Ten g of WS and 100 mL of CuSO4 aqueous solution (0.07 M) (pH between 6 and 9) were mixed in the flasks and at 50ºC for 2h, followed by evaporation of water in a rotary evaporator. The reduction of CuO was carried out by the drop-wise addition of 0.25 g NaBH4 to 70 mL ethanol 96% solution of obtained solid, and then, intensive stirring was continued for 3h. After filtering and washing the mixture, CuO NPs were loaded on WS. Then microscopic TEM was used for characterization of CuO NPs coated on WS.
3.4. Plant Extract
The fresh aerial parts of Aloe vera were collected, washed, chopped, and shaded to be dried. We used hydroalcoholic and percolation method for plant extraction in this experiment. In brief, hydroalcoholic (50% ethanol and 50% distilled water v/v) extract of the dried and chopped plant was provided using three steps of percolation method, and then, the extract was evaporated at temperature 40°C to dry. The final extract was stored at -20°C without exposure to light.
3.5. K562 Cells Culture
The K562 erythroleukemia cell line was purchased from the Pasteur Institute of Iran. Cells were cultured in RPMI 1640 media, supplemented with 10% fetal bovine serum (FBS) and penicillin plus streptomycin (100 IU/mL and 100 μg/mL, respectively). The K562 cells were cultured in a 5% CO2 humidified atmosphere at 37°C to reach 80% confluency.
3.6. PBMC Isolation
Heparinized blood samples (20 mL) were gathered from five volunteers. Samples were centrifuged at 400 g at 4ºC for 10 min to isolate a buffy-coat layer. The collected cells were diluted 1:2 in PBS and centrifuged over Histopaque 1083 for 30 min at 800 g in 18ºC. The cells were re-suspended and hypotonic lysis was used to remove contaminant erythrocytes. Subsequently, PBMCs were washed three times and then re-suspended in RPMI, which contained 10% fetal bovine serum.
3.7. Cell Treatment
The K562 cells or PBMCs were plated in 96-well flat-bottomed plates (1×105 cells/200 μL/well) and incubated for 24h with serial dilution of Aloe vera extract (0, 20, 40, 60, 80, 160 and 320 μg/ mL) or serial dilution of Green CuO NPs (0, 50, 100, 200 and 400 μM).
After incubation, cultures were pulsed with 20 μl of a 5 mg/mL solution of MTT in PBS at 37°C for 4h. Then, the medium was removed, and 150 mL DMSO was added. The plate was shaken vigorously to dissolve Formosan crystal, and the optical density (OD) was measured using a microplate reader (Dynatech, Denkendorf, Germany) at 550 nm. The experiments were performed in triplicate, and the results were reported as the survivability present according to the ratio of (OD550 of treated cells to OD550 of non-treated cells) × 100. The inhibitory 50% concentration (IC 50) was determined by linear regression. In another experiment, the K562 and PBMC cells were treated with a combination of Aloe vera extract, and Green CuO NPs at minimal cytotoxic concentrations, and the inhibitory effects were estimated.
3.8. Statistical Analysis
The IC50 was assessed by linear regression analysis using GraphPad Prism (version 8.0.2.263). Statistical analysis was done using one-way ANOVA and Tukey’s post hoc tests. Values of P < 0.05 were considered statistically significant.
4. Results
4.1. Characterization CuO NPs Coated on WS
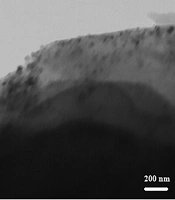
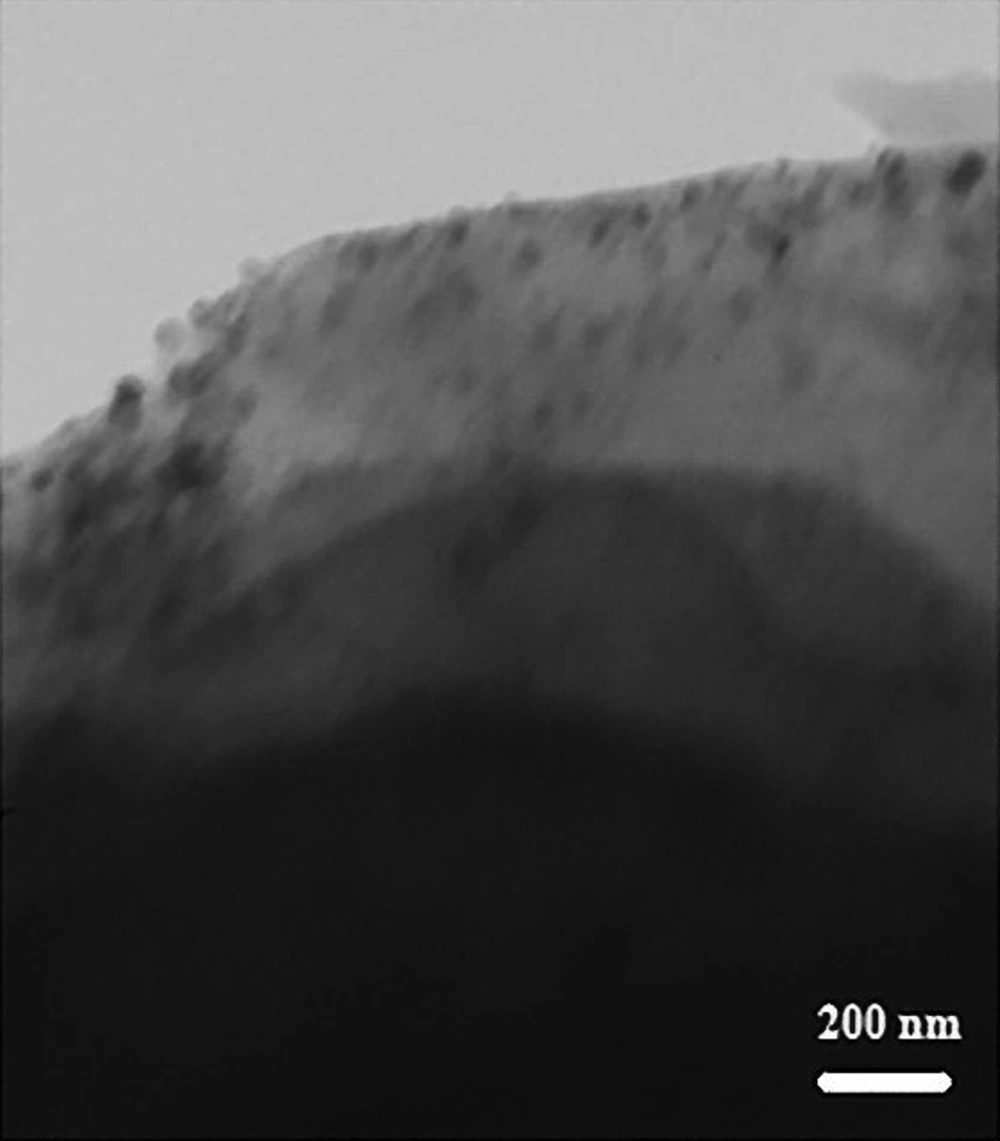
Results of atomic absorption spectroscopy (AAS) analysis exhibited that the concentration of Cu NPs on the walnut shell is 3.03%. Transmission electron microscopy (TEM) image of the as-prepared material showed 60 - 80 nm particles (Figure 1).
4.2. The Results of Effects of Hydroalcoholic Extract of A. vera and Green CuO NPs on the Cancer Cell and Normal Cells
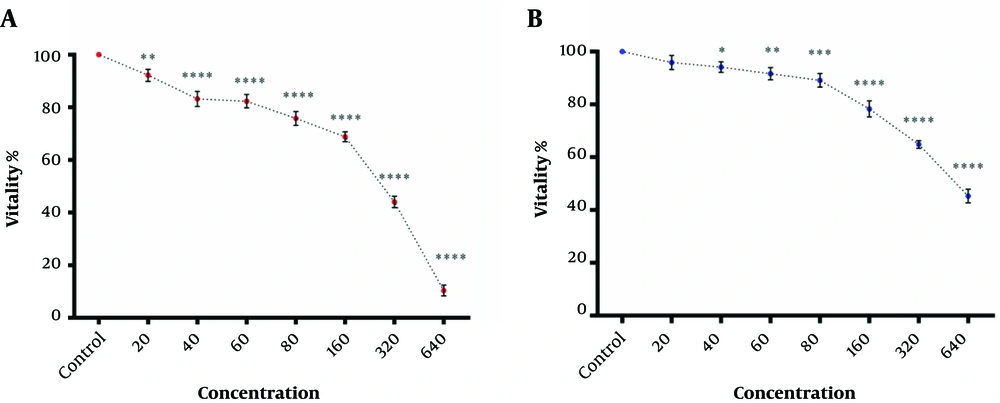
As shown in Figure 2A, hydroalcoholic extract of Aloe vera had cytotoxic effects against the K562 cell line in a dose-dependent manner. Although the hydroalcoholic extract of Aloe vera also showed cytotoxic effects against PBMCs (Figure 2B), the IC50 value of hydroalcoholic extracts of Aloe vera against the K562 cell line was significantly smaller than the IC50 value of extracts against PBMCs, as shown in Figure 2A and 2B (323.9 ±5 μg/ mL vs. 550.54 ± 6.2 μg/ mL).
The cytotoxic effect of Green CuO NPs against K562 cells and PBMCs was somewhat dose-dependent, not completely similar to the hydroalcoholic extract of Aloe vera (Figure 3A and 3B). Nevertheless, the IC50 value of the Green CuO NPs against K562 cell line had no significant difference compared to the IC50 value of the Green CuO NPs against PBMCs (175 ± 9 μM vs. 186.89 ± 8.88 μM) (Figure 3A and 3B).
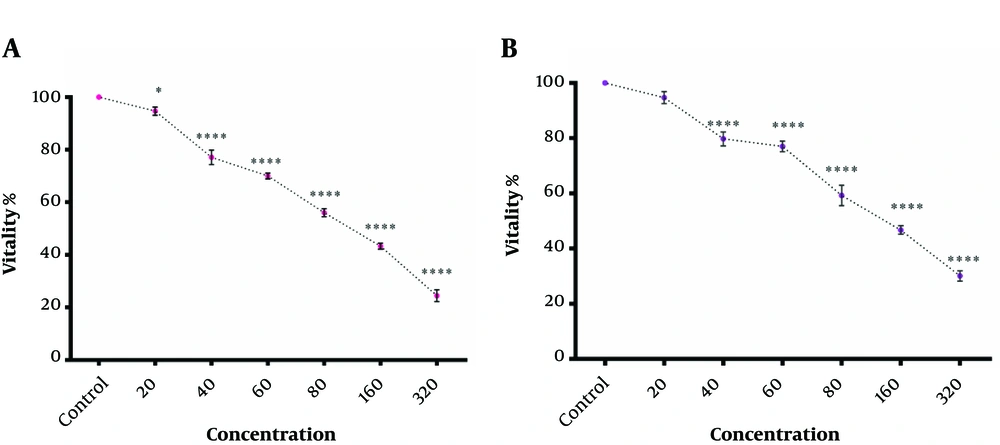
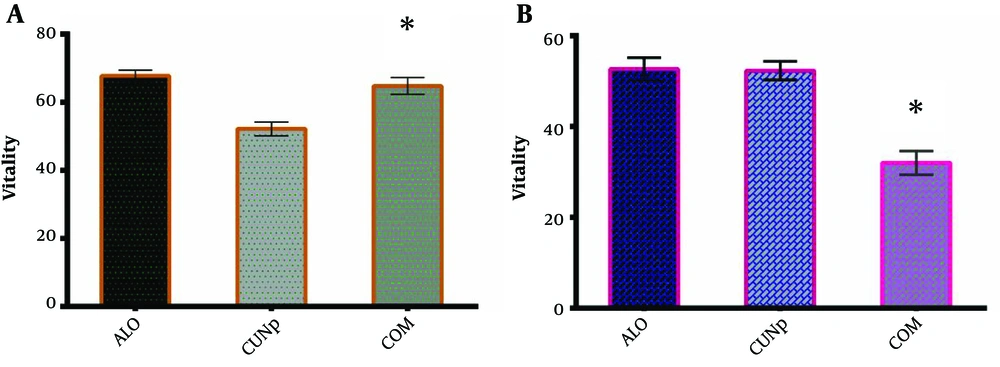
In the following, a combination of two factors at IC50 concentration was used. As shown in Figure 4, this combination had provided synergistic benefits and lead to more cytotoxicity against K562 cancer cells than their individual or separated treatment of K562 (Figure 4A). Moreover, attained results showed that combined hydroalcoholic extract of Aloe vera and green CuO NPs at each IC50 concentration had not shown a more pronounced cytotoxic effect against PBMCs compare to Green CuO NPs. However, the combined cytotoxicity against PBMCs was similar to the cytotoxic effect of hydroalcoholic extract of Aloe vera (Figure 4B).
5. Discussion
The main bioactive compounds of the Aloe plants were being anthraquinones, and enzymes (including bradykininase and cyclooxygenase), as well as other composites like amino acids, saccharides, and vitamins (19). It has been demonstrated that Aloe-emodin had significant cytotoxic and anticancer benefits in contradiction of neuroectodermal malignant tumor (30), hepatoma cells, and lung squamous cell carcinoma (31). Also, recent data showed that the extracts of Aloe vera could induce apoptosis in hepatocellular carcinoma (HepG2), cell cutler, by increasing P53; meanwhile, decreasing Bcl-2 gene expression.
Traditional anticancer agents have many side effects and caused a dramatic death in the normal cell, such as hematopoietic and immune cells in addition to the cancer cell (2). Here, we used PBMCs as a normal cell for evaluation of unwanted side effects. Attained data in a recent study indicated the IC50 value of the hydroalcoholic extract of Aloe vera against the K562 cell line was significantly less than an IC50 value of the hydroalcoholic extract of Aloe vera against PBMCs. It is clear that an agent with minimal cytotoxic effects against healthy cells and maximal cytotoxics effect against tumor cells is more favorable of the dose-dependent cytotoxicity of CuO NPs in contrast to cancer cell lines as was described beforehand (8). Previous studies advocated that CuO NPs caused cytotoxicity in mammalian cells through oxidative stress in a dose-dependent manner (2, 28). DNA damage is due to oxidative stress but, there is little information about practically the genotoxicity of Green CuO NPs exposure to human cells (26). It is suggested that the Cu NPs, by inducing apoptosis, have cytotoxic effects against Hela cells of human histiocytic lymphoma, human cervical cancer origins, and U937, respectively (28). Here, we showed that Green CuO NPs had cytotoxic effects against the K562 cell line. Of note, the IC50 value of the green CuO NPs against the K562 cell line had no significant difference compared to the IC50 value of the Green CuO NPs against PBMCs. Therefore, unlike Aloe vera extract, the marginal safety of Green CuO NPs is low.
Diverse classes of chemical cytotoxic drugs are accepted for the control of malignancy. Nevertheless, these drugs have very important side effects because of low specific toxicity as well as numerous patients have a fewer than desirable response (32). On the other hand, because of genetic instability, cancer pathology is very complex. Also, selective pressure by a single drug can select drug-specific tumor resistant cells (33). Then, it is difficult for a single drug to be valuable in all malignancy throughout all periods of the syndrome. One appropriate approach to control malignancy is to identify a coherent mixture of new medications or existing agents. Combination therapy by selective therapeutic agents potentially regresses drug resistance, while simultaneously promote anticancer benefits. The aim of combination therapy in cancer is that the blend regresses the tumor growth rate better than treatment with either agent alone. On the other hand, it is necessary that combination remedy has a good well-being margin and does not construct additional toxicities when used in amalgamation. In modern medicine, many studies have been designed on the therapeutic potentials of plant extracts and NPs. Interestingly, a recent report showed that combined silver NPs and extracts of the medical plant Chinese honeysuckle, Lonicera japonica Thunb had enhanced antimicrobial action against pathogenic Escherichia coli CMCC44113 (34). Also, it has been shown that curcumin (a natural phenol) and temozolomide overloaded magnetic NPs had profound cytotoxic influence in glioblastoma spheroid model (35). Earlier studies have analyzed Aloe vera and other substance effects on malignancy cells. The cytotoxic prospective of Aloe vera crude extract (ACE) by themselves or in mix with cisplatin in cervical (HeLa) and human breast (MCF-7) malignancy tumor cells demonstrated the effects were associated with the downregulation of cyclin D1, CYP 1A2, CYP 1A1, and increased manifestation of p21 and Bax in MCF-7 and HeLa line cells. Moreover, a combination of cisplatin and ACE affirmed a blend indicating synergistic development inhibition set alongside the agents applied specific (36).
Here, we used the IC50 dose of each agent against K562 in combination. Fortunately, combined agents at each IC50 concentration had not shown a more pronounced cytotoxic effect against PBMCs compared to Green CuO NPs and had similar cytotoxic effects against PBMCs compared to the cytotoxic effect of the hydroalcoholic extract of Aloe vera. Moreover, the findings indicated that combined treatment provides synergistic benefits and lead to more cytotoxic effects against the K562 cell line than their individual treatment of K562.
Low-cost agricultural byproduct and waste in the walnut processing industry is one of the most commonly used renewable resources, and it should serve as a useful starting point for further research (37). It has been well acknowledged that natural products played serious roles in current drug development, mainly for antitumor agents and antibacterial. Even at the beginning of the 21st century, 11% of the 252 drugs, which considered rudimentary and critical by the WHO, were wholly with flowering plant origin (37). Some components of Aloe vera, like anthraquinones are genotoxic (19), on the other hand, CuO NPs could induce DNA damage due to oxidative stress (26). Based on the results, it seems that the combination of two agents can promote a beneficial synergistic effect. This study is a preliminary study. Nevertheless, this study is a preliminary survey. Therefore, the active ingredients of each plant should be determined and then by combining the active ingredients in pharmaceutical compounds as drugs for use in patients. Then, based on all the active mechanisms, combinations of two or three monomers should be screened out using the network pharmacology technique. Subsequently, through structural changes and advancements as well as altering the dose and proportions of the species used, the effectiveness of the drug group will be continually improved; side effects will be continually reduced, in a way that, finally, the optimal synergistic drug blend will result.
Hence, the renewed attentiveness in the development of natural goods requires the confluence of the current methods and harmonization of regulations associated with their research and development between different fields of science.
5.1. Conclusion
The combined walnut shell-extracted CuO NPs and hydroalcoholic extracts of Aloe vera provide more favorable cytotoxicity against K562 cell line whiles, without any additive cytotoxicity against PBMCs. Therefore, the combinative of Aloe vera and green CuO may be considered an alternative or supplement for cancer therapy later.