1. Background
Schizophrenia is a heterogeneous syndrome without one clear definition of a sign or symptom and is unidentifiable with any known diagnostic laboratory tests [1]. Indeed, schizophrenia is a neural development disorder that may relate to several genetic and environment factors such as birth complications, the urbanization and season of birth [2]. Schizophrenia is characterized with several symptoms such as: positive symptoms, negative symptoms and cognitive deficiency [3]. Positive symptoms (such as delusions, hallucinations), negative symptoms; including reduction or loss of normal function and cognitive deficiency involve defects in the processing of executive functions, attention, verbal memory and learning [4]. Prevalence average of this disorder is about 1% of global scale [5]. Also, bipolar disorder is a chronic neurological abnormality. Affected patients expose mania and hypomania episodes. When depressed, patients can suffer from loss of energy, lack of interest and poor concentration. During the manic episodes, they may become hyperactive and irritable [6]. Average prevalence of this disorder is about 1% of global scale [7]. The heritability of bipolar mood disorder has been estimated 80 - 90% [8]. Also, the heritability of schizophrenia has been reported 80% [5]. Neurochemical studies have shown that various neurotransmitters, such as dopamine, glutamate, serotonin and GABA, may be involved in the molecular mechanisms of both diseases [9-12]. Glutamate is an excitatory neurotransmitter that is involved in a variety of neural processes including neuronal toxicity, synaptic flexibility and inclusive neuronal development [13]. Normal glutamatergic neurotransmission involves glial cells, pre and post synaptic neurons, enzymes, transporters and glutamate receptors. Disrupting any of the cases, may be lead to disruption in the normal glutamatergic neurotransmission that could be involved in neurotransmission disturbances [14]. NMDA receptor is a post synaptic glutamate receptor (GluRs) [15]. NMDA receptor antagonists can create some of the positive and negative symptoms of schizophrenia [16, 17]. One relatively new candidate gene D-amino acid oxidase activator has recently gained attention. Not only because it has shown association with bipolar disorder and schizophrenia, but also for the important role of this gene in glutamatergic neurotransmission. Understanding of the function of this gene could therefore contribute to our understanding of the etiology of these diseases [18]. DAOA gene (GenBank accession no. NC-000013) was considered by Chumakov et al. initially [19], and then a series of association studies showed correlation between DAOA variations with schizophrenia and bipolar mood disorder [20, 21].
Chumakov et al. mapped the G72 gene to chromosome 13q34. The functional mechanisms of DAOA/G72 are still not fully understood. Chumakov et al. showed that the G72 protein activates DAAO protein (D-amino acid oxidase, 12q24), that is involved in the D-serin mechanisms [19]. Subsequently, G72 was given the designation D-amino acid oxidase activator (UCSC genome browser, Ensembl genome browser, National Center for Biotechnology Information [NCBI]).
2. Objectives
The purpose of the study was determination of genetic Overlap between affective disorders using Association Analysis of M18 and M23 SNPs of DAOA/G72 gene with schizophrenia and bipolar disorder.
3. Patients and Methods
3.1. Sampling
In this case-control study blood was taken from 127 healthy controls and 100 patients with schizophrenia and 100 patients with bipolar disorder with the consent from each subject or subject’s guardian in Ahvaz. Schizophrenia patients had mean ± SD age 27.4 ± 8.2 years. Patients with bipolar disorder had mean ± SD age 24.4 ± 7.5 years. Healthy controls had mean ± SD age 38±7 years. Patients were diagnosed according to criteria of Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). All patients had a history of at least two previous hospitalizations. Selection of the control group was performed by the General Health Questionnaire (GHQ) [22] scaled 28 among non-dependent individuals in Ahvaz blood transfusion service. This screening questionnaire is commonly used in general community or non-psychiatric patients, to screening patients with psychiatric disorders from health controls. This questionnaire has four subscales including: physical symptoms, anxiety and insomnia, social dysfunction disorder and depression. Assign at least one deterioration of subscales, were led to removal of the person from the healthy population. Patient and control groups were matched for gender. There was no significant difference in sex distribution between cases and controls (67% male in schizophrenia patients, 47% male in bipolar disorder patients, 55% male in controls).
3.2. DNA Isolation
Genomic DNA was extracted from white cells of peripheral blood collected in EDTA tubes. A salt precipitation method was used to extract DNA from each sample.
3.3. SNP Genotyping and Statistical Analyses
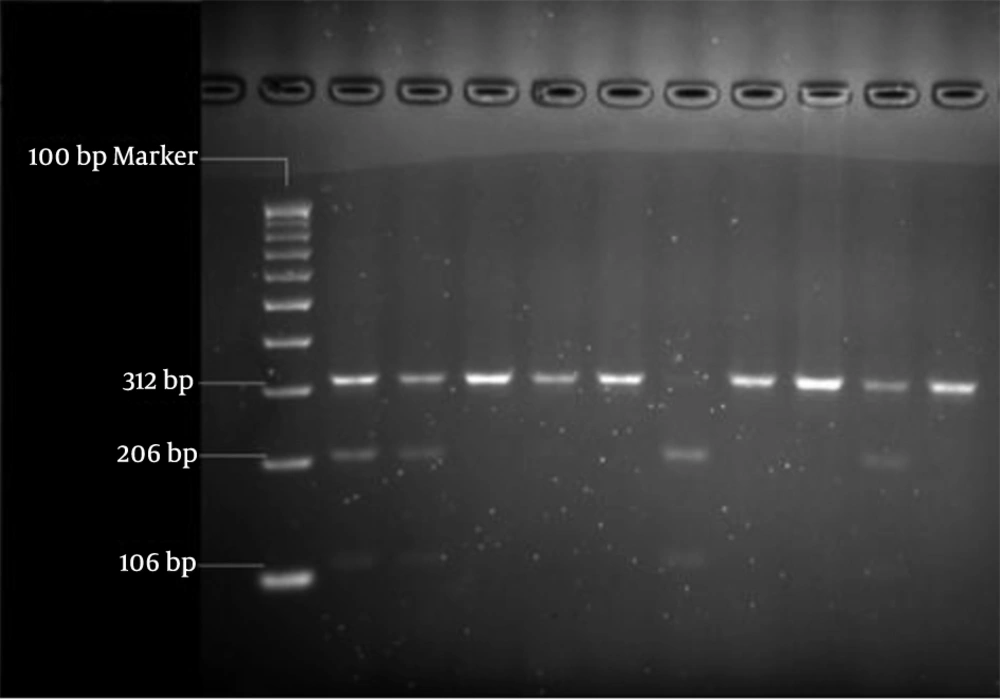
We selected single nucleotide polymorphisms from the public SNP database, dbSNP (http: //www.ncbi.nlm.nih. gov) and from published finding. We chose the markers M18 (rs947267) and M23 (rs3918342), because the M18-M23 haplotypes C-A and C-T have recently been associated with schizophrenia and bipolar disorder (Table 1). As depicted in Table 2, the samples were amplified by two primer pairs. Then RFLP was performed to determine genotypes of two polymorphisms M18 and M23 by HaeIII and BsaAI restriction enzymes (Figures 1, 2). Also, the data was confirmed by sequencing assay. Depending on the M18 and M23 markers, the subjects were separated in three groups: high risk, intermediate risk and low risk. The data were analyzed by Logistic Regression and Mantel-Haenszel χ2 tests.
| SNPs | Position | Function | Alleles |
|---|---|---|---|
| 106139662 | Intronic | A/C | |
| 106185749 | Upstream 5′UTR | C/T |
| Forward: 5'-GGAAACCAGAAG GTGAAA-3' | |
| Reverse: 5'-GAATCAGAAAGG AAAAGTGT-3' |
4. Results
Depending on the M18 and M23 markers, the subjects were separated in 3 groups (Tables 3 and 4). In conclusion; according to this data (P = 0.09) we did not find any association between M18 and schizophrenia, but according to (P = 0.001) we found a significant association between M23 and schizophrenia. Also we found a significant association between both of two SNPs, M18 (P < 0.001) and M23 (P < 0.001) with bipolar mood disorder. Subjects who had a homozygote A-allele on M18 and homozygote T-allele on M23 were classified as “high risk”. All other subjects were classified as intermediate risk group.
| SNPs | Genotypic Distribution | Schizophrenia, % | Bipolar Disorder, % | Controls, % |
|---|---|---|---|---|
| CC | 12 | 2 | 22.85 | |
| AC | 49 | 57 | 46.45 | |
| AA | 39 | 41 | 30.7 | |
| CC | 17 | 22 | 4.725 | |
| TC | 37 | 55 | 42.52 | |
| TT | 46 | 23 | 52.755 |
| SNPs | Genotypic Distribution | Schizophrenia | Bipolar Disorder | Controls |
|---|---|---|---|---|
| C | 0.365 | 0.305 | 0.54 | |
| A | 0.635 | 0.695 | 0.46 | |
| C | 0.355 | 0.495 | 0.26 | |
| T | 0.645 | 0.505 | 0.74 |
5. Discussion
According to the results of this study, DAOA gene is discussed as an overlapping gene in susceptibility to both schizophrenia and bipolar mood disorder in southwest Iran. Moreover, our results and previous studies provide some support for role of glutamatergic pathway genes in susceptibility to affective disorder in southwest Iran. Overall, our data provides further evidence for a positive association between the DAOA locus and schizophrenia and bipolar disorder. A meta-analysis [23] was conducted; combined 18 association articles have shown association between M18 and schizophrenia. This association was not observed in Iran. Also, in this meta-analysis there was no association between M18 and bipolar disorder, while there was a significant association between M18 and bipolar disorder in Iran. Consequently, the association between M18 with schizophrenia and bipolar disorder is quite different in southwest Iran from Asian population. Moreover, a significant association has been observed between M23 and both of schizophrenia and bipolar disorders in the UK [24].
Among other studies of glutamatergic pathway, genes that have shown association with schizophrenia in Iran include NRG1 gene [25], dysbindin gene [26], GRIN1 gene [27] and DTNBP1 gene [28]. Schizophrenia and bipolar mood disorders affect many families simultaneously. This theme suggests that these disorders have a shared genetic etiology at least to some extent [29]. Traditionally, search for predisposing genes, has proceeded under the presumption that schizophrenia and bipolar disorder are segregate disease entities. But, a twin study [30] has added to previous evidence challenging this presumption [31]. Although schizophrenia and bipolar disorder are defined as distinct and exclusive diagnostic entities, they show an overlap of symptoms [32]. Hence, the separation of these syndromes into etiologically homogenous subtypes is currently under debate [7].
In addition, genetic linkage analysis has identified several overlapping regions in disorders, including chromosome 6p, 13q, 18q and 22q [33]. Also, several recent association studies suggest that variation in DAOA/G72, DISC1 and NRG1 genes implicated in influencing susceptibility to schizophrenia also influences susceptibility to bipolar disorder [34]. The best supported gene from recent studies of bipolar disorder is DAOA, which was originally identified in studies of schizophrenia. In summary, we genotyped two SNPs of DAOA in a control study of Iranian population. In conclusion, these results may provide further support for genetic overlap in DAOA gene between schizophrenia and bipolar disorder.