1. Background
Toxoplasma gondii is an obligatory intracellular protozoan detected worldwide (1). Its definitive hosts are felids; however, its intermediate hosts are approximately all warm-blooded animals. Moreover, it could parasitize all the nucleated cells of its hosts; therefore, T. gondii is one of the most successful parasites in the world (2). Toxoplasma gondii can be transmitted to humans by the ingestion of tissue cysts in raw or undercooked meat, accidental consumption of oocysts excreted from cats in the environment, and congenital transmission by tachyzoites (3).
During the acute phase of the T. gondii infection, tachyzoites multiply rapidly within the nucleated cells; finally, they lyse host cells and are hematogenously distributed throughout the body. After the acute phase, the host immune system develops a protective response; finally, it is altered from tachyzoite to bradyzoite form, and the formation of cysts occurs (4, 5)The parasite generates cysts in muscle and neural cells, including neurons, glial cells, and especially astrocytes (6), because the brain is an immune-privileged site for having a long life of T. gondii cysts (7).
The parasite does not usually cause much damage to immunocompetent hosts; however, it has been associated with cognitive impairments, emotional disturbances, and schizophrenia (8). Recent studies have shown that the transmission of T. gondii is also facilitated by the ability to modify its host’s behavior (9-11). According to the manipulation hypothesis, specific parasites can change host behavior for their own benefit (1). Toxoplasma gondii-infected rats and mice had a less innate fear of the odor of cat urine; this effect is useful for the parasite (1). The mechanisms for these changes have not been clearly identified to date; however, there is evidence that toxoplasmosis increases dopamine levels in the amygdala in the brain of infected mice by genes encoding the tyrosine hydroxylase enzyme production (12). It is noticeable that the hippocampus and amygdala are structures in the brain associated with natural defense behaviors and emotion processing (13).
During the acute phase of infection, the host immune system activates and raises the level of cytokines, including interleukins (i.e., interleukin-1 and interleukin-6), tumor necrosis factor-alpha, and interferon-gamma, that may indirectly alter brain neurotransmitter levels (14). Cortisol is a glucocorticoid hormone secreted by the adrenal cortex. The glucocorticoids could synthesize the proteins that may have inhibitory or stimulatory effects on some specific tissues (15).
2. Objectives
The present study mainly aimed to investigate the effect of T. gondii infection on anxiety in an animal model and determine the levels of cortisol and interleukin-17 (IL-17) in rats.
3. Methods
3.1. Toxoplasma gondii Strain Culture
This study was designed and carried out in the Research Laboratory of the School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran. Toxoplasma gondii RH strain was donated by the Department of Parasitology, School of Public Health, Tehran University of Medical Science, Tehran, Iran. The tachyzoites of this strain are lethal to mice during 4 - 5 days after inoculation. The obtained parasites were reproduced by intraperitoneal (IP) inoculation in BALB/c mice and used 3 days after the inoculation of tachyzoites for this experiment. The collected tachyzoites were suspended in sterile phosphate-buffered saline (PBS) and counted the number of tachyzoites; then, peritoneal fluid was mixed with PBS and was counted using a Neubauer chamber. For the prevention of bacterial growth in the peritoneal exudates, penicillin and streptomycin were added (1000 units of penicillin and 1 mg of streptomycin/per ml of IP exudates).
3.2. Experimental Animals
A total of 40 male Wistar rats weighing 200 - 250 g were obtained from the Animal House of Hamadan University of Medical Sciences. The rats were randomly allocated to four groups (n = 10 for each group), namely uninfected animals as the control group, infected group, infected and trimethoprim-sulfamethoxazole (TMP-SMX)-treated group, and infected and dexamethasone-receiving group. Food and water were freely available equally for all the groups, and the room temperature was maintained at 22 - 25ºC. The light was on from 7 a.m. to 7 p.m.
3.3. Toxoplasma gondii Infection of Rats and Drug-Treated Animals
The rats of the control group were injected with 0.2 ml PBS (group 1). The experimental group rats were infected by the IP injections of 106 tachyzoites (group 2) (16). The treatment of animals started 5 days after the infection for 10 consecutive days with the IP doses of 0.5 mg/kg/day TMP-SMX (group3) (17). The animals were intramuscularly injected with dexamethasone at 1.5 mg/kg/day for 7 days immediately after parasite inoculation (group 4) (18).
3.4. Test for Anxiety Evaluation
The plus maze was made of wood with two opposite open arms (50 × 10 cm) and two opposite enclosed-end arms by 40-cm height walls. The arms were connected by a 10 × 10 cm central square; accordingly, the maze formed a ‘plus’ shape. The maze was elevated 50 cm above the floor. A camera was vertically mounted above the maze, and the behavior was visible from a screen monitor in an adjacent room. The changes in the spent time in the open arms and entries to the open arms are indicated as anxiety; however, the number of closed-arm entries is the best indicator of normal activity in the maze. The behavior test was performed within 8 to 12 a.m. Each rat was placed in the central square of the plus maze facing a closed-end arm. The total activity unit of rats was the mean of the number of entries to plus maze arms in each group. The measurements were performed by RAT IL-17 PicoKine ELISA Kit (Boster Bio, USA) and Monobind Cortisol Kit (CAT NO: 3625-300, Monobind Inc., USA) according to the manufacturer’s instructions.
3.5. Statistical Analysis
Statistical analyses were performed using SPSS software (version 16). Behavioral scores were analyzed using a one-way analysis of variance followed by Tukey’s post-hoc test. Differences were considered statistically significant if p-values were less than 0.05. The relationship between cortisol and IL-17 concentration with behavior scores was assessed by the Pearson linear correlation.
3.6. Ethical Considerations
This study was approved by the Research Ethics Committee of Hamadan University of Medical Sciences (ethics code No.: IR.UMSHA.REC.1394.168).
4. Results
4.1. Behavioral Tests
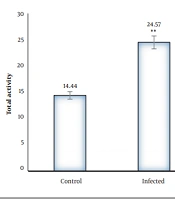
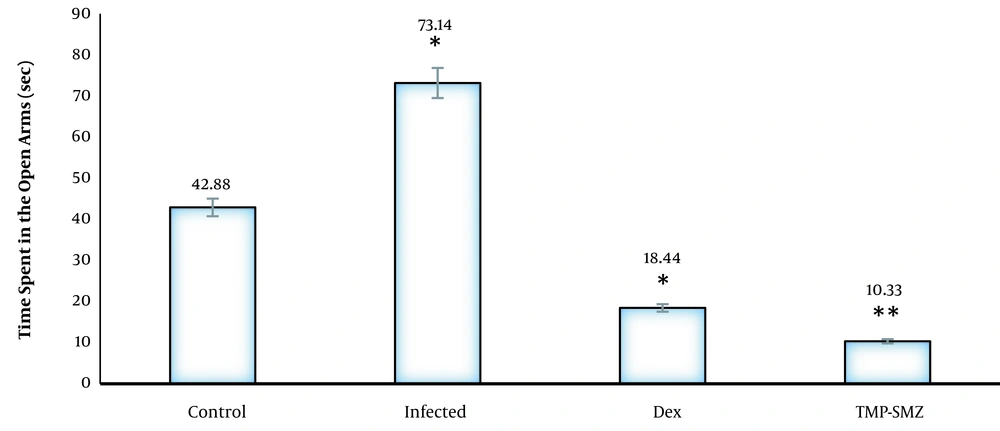
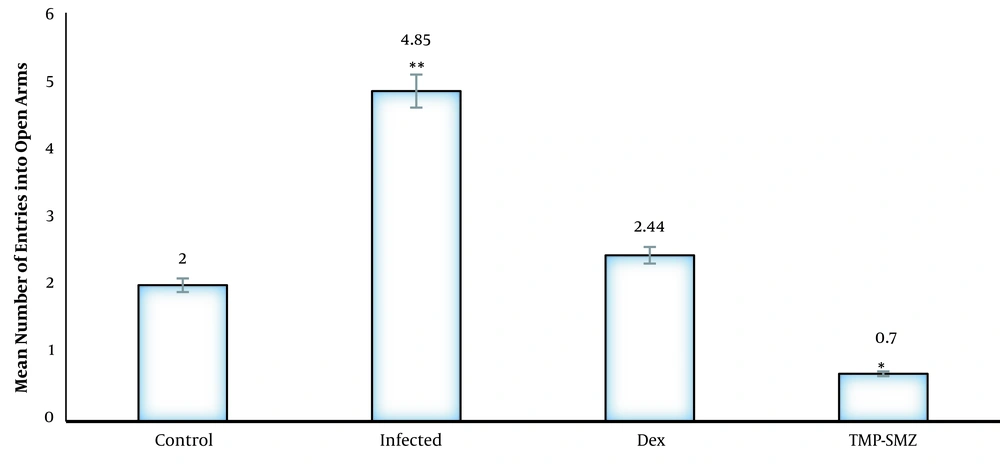
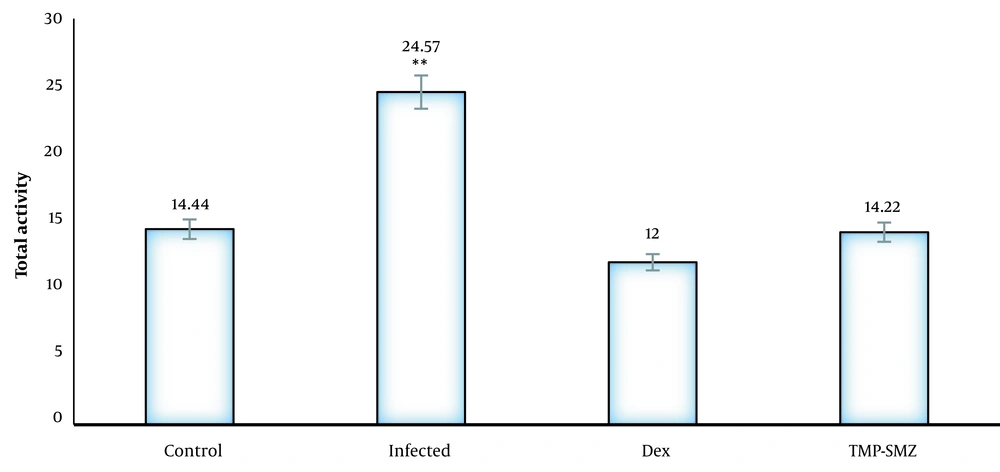
In the elevated plus maze, the infected rats had a significantly higher number of entries to the open arms, and the mean spent time in the open arms was higher than that of the control group (P < 0.01). The dexamethasone-receiving and TMP-SMX-treated rats had a lower number of entries to the open arms, and the mean spent time in the open arms was lower than that of the infected group; nevertheless, there were no significant differences in closed arm entries between different groups with the control group (Figures 1 and 2). Regarding the total activity, the infected rats had significantly higher levels than the controls (Table 1), dexamethasone-receiving rats, and TMP-SMX-treated rats; however, the differences were not statistically significant (Figure 3).
Effects of Toxoplasma gondii infection and treatment of infected rats by dexamethasone and trimethoprim-sulfamethoxazole (TMP-SMX) on spent time in open arms during a 10-minute test in Wistar rats; results presented as mean (± standard deviation); * P < 0.05 and ** P < 0.01 in comparison to control.
Effects of Toxoplasma gondii infection and treatment of infected rats with dexamethasone and trimethoprim-sulfamethoxazole (TMP-SMZ) on mean number of entries to open arms during a 10- minute test in Wistar rats; results presented as mean (± standard deviation); * P < 0.05 and ** P < 0.01 in comparison to control.
| Groups | Closed Arms | Open Arms | Total Activity |
|---|---|---|---|
| Control | 12.66 | 2 | 14.44 |
| Infected | 20 | 4.85 | 24.57 |
| Dexamethasone | 9.55 | 2.44 | 12 |
| Trimethoprim-sulfamethoxazole | 13.88 | 0.77 | 14.22 |
4.2. Hormonal Assay
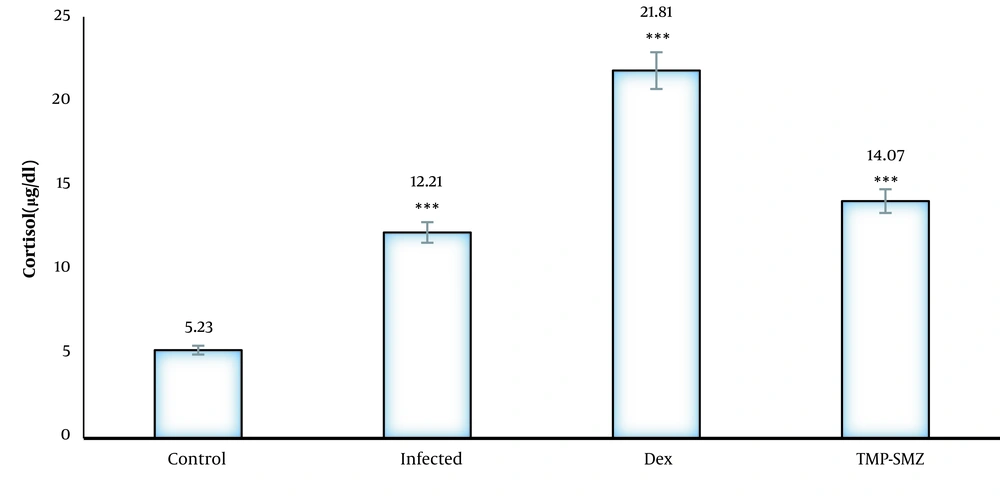
As depicted in Figure 4, the cortisol serum level is significantly higher in the infected rats than that in the healthy rats. The increased levels of cortisol in the dexamethasone group could be due to the treatment effect.
4.3. Serological Test
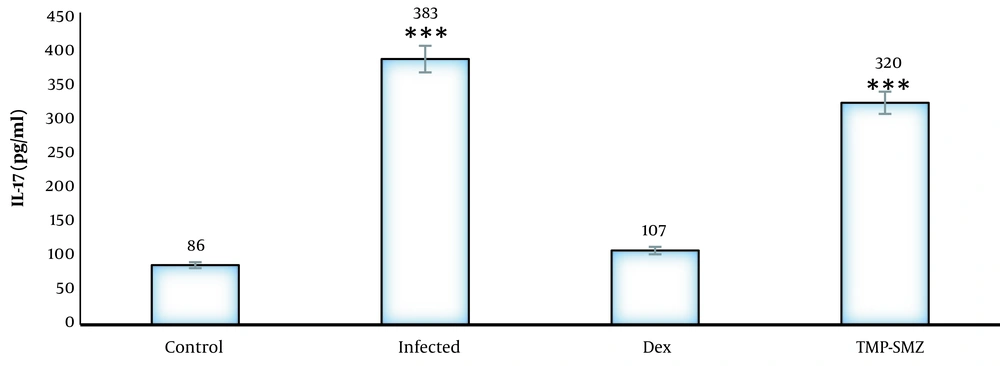
As indicated in Figure 5, the IL-17 of serum was significantly higher in the infected rats than that in the healthy rats.
5. Discussion
The rats infected with T. gondii had an increased spent time in the elevated plus maze, number of entries in the open arms, and total activity (increase in total activity probably due to the increased number of entries in the open arms). In contrast, the dexamethasone-receiving and TMP-SMZ-treated rats had decreased spent time and numbers of entries in the open arms in comparison to the infected rats, and there was no significant difference in total activity in comparison to that of the control group. The results showed the anxiolytic effects of T. gondii with 106 tachyzoites in the two-week post-inoculation period in rats. In this study, a rat model was used because mice are more sensitive than rats to T. gondii infection, and after a few days of suffering severe brain damage they die due to disseminated acute infection.
Previous studies suggested that not only infected rodents reduced normal aversion to cat odor but also they had a special attraction to it (19). According to a study performed by Zareian et al. (20), there was no significant difference in the anxiety levels between infected and healthy rats; however, the present study showed that T. gondii infection reduced the levels of anxiety in rats. A study conducted by Hay et al. (21) reported a reduction of open arm exploration and total activity counts in mice. Gonzalez et al. (22) showed an increased open arm exploration; nevertheless, it did not change total activity in rats infected with T. gondii.
Kannan et al. (23) indicated the evaluated effects of T. gondii infection on the mice behavior (i.e., 100 and 1000 tachyzoites) by two type II strains of T. gondii, Prugniaud (PRU), and ME49. The increased activity of locomotors was observed in PRU-infected mice in the open field in contrast to ME49-infected mice, and a significant decrease in Y maze entries was observed showing the impaired spatial memory of mice. In another study conducted on pregnant women seropositive for toxoplasmosis, a direct correlation was reported between anti-T. gondii immunoglobulin G and anxiety level (24).
In several studies, behavioral changes have been argued by the pattern of immune response due to increased levels of inflammatory cytokines during the acute phase of infection, probably increasing the permeability of the vessels that the blood supply to the damaged organ in the brain (25). In the present study, the levels of pro-inflammatory IL-17 in infected rats significantly increased, compared to those of the healthy rats. Glucocorticoids are the most important modulators of immune responses and play an important role in systemic stress responses. They are known for immunosuppressive and anti-inflammatory properties in different diseases (18). The results of the current study showed that the treatment of infected rats with dexamethasone improved the anxiolytic effects of parasites.
Toxoplasma gondii alters important parameters that are responsible for energy homeostasis in the brain. Acetylcholine is an important neurotransmitter in the brain, and changes in its activity are associated with neurological damages. Toxoplasma gondii increases the level of acetylcholine in the brain. The TMP-SMX can adjust acetylcholinesterase activity in infected rats (17, 26). In the present experimental rats treated with TMP-SMZ, there was a significant reduction in the spent time in the open arms, even compared to that of the non-infected rats, which is probably caused by the treatment effects.
Other candidate mechanisms for behavioral changes include decreased glutamate/gamma-aminobutyric acid ratio in limbic areas after astrocytes damage, increased levels of dopamine in the brain, neurotransmitter alteration, and release of anxiolytic-like compounds by the parasite and hormonal manipulation (22, 27). Mahbodfar et al. (28) demonstrated that the concentrations of testosterone and cortisol increased in individuals seropositive for toxoplasmosis. Moreover, in this study, the infected rats had high levels of cortisol than the healthy rats. Although corticosteroids do not directly affect behavior, they had an important role in fear and anxiety control centers (29). Cortisol levels increase through tachyzoites, cysts, and cystozoites in infected individuals (18, 30, 31).
5.1. Conclusion
In conclusion, this study indicates that T. gondii has anxiolytic effects on rats, and IL-17 and cortisol levels increased in the serum of infected rats. The treatment was not different between the rats receiving the anti-inflammatory agent and healthy rats.