1. Background
Biological scaffolds that compose extracellular matrix (ECM) can facilitate the restructuring of a large number of pre-clinical studies in animal tissues and also in clinical applications. ECM as a scaffold is produced from different tissues such as blood vessels, skin, small intestinal sub mucosa, bladder and liver [1, 2]. Composition of ECM as a scaffold includes different mixture of molecules. The proteins, where attached to cell surface, strengthen the matrix [3]. As we know interaction between cells and ECM provides suitable three-dimensional structures. In addition, it provides signaling pathways for cell behavior such as migration, differentiation, adhesion and proliferation which are important for tissue development, healing, organ development, repair and physiologic regeneration. This provides a promising alternative to synthetic scaffolds and a foundation for regenerative efforts [4, 5]. Therefore, we can use extracellular matrix from different tissues to study cell behavior interaction in vitro. For this purpose, decellularized methods are used through elimination effect of cells on extracellular matrix. As a result, the remained extracellular matrix, can be used as a scaffold [6]. Langer and Vacanti defined tissue engineering as a new field of study in which the principles of engineering and biology are applied to improve the damage tissue, renew the repair and preserve the tissue function [7].
Tissue engineering progressed rapidly in the 1990s. During that period, the subject of alternative biological tissues was expressed to regenerate tissues and nowadays can be introduced as a promising technique for the development of biological alternative that can store, maintain or improve the function of tissue.
Creation of the human tissue engineered products, production of autolog somatic cells or mature stem cells with or without any biocompatible matrix is a new approach to solve loss of tissue and organs transplant [8]. Urinary tract and bladder are susceptible to a variety of pathological conditions from fetal development period to adulthood. A number of synthetic materials and natural tissues have been investigated for the reconstruction of functionally deficient bladders. These natural materials include: bladder allograft, skin, muscle, gastric-intestinal parts, placenta, small intestinal sub mucosa and the decellularized matrix of bladder [9]. Apart from substrate, the type of cell sources has an important role in the study of cell behavior during the interaction with the substrate. The cell sources that are usually used to study are fibroblast, stem cells and cancer cells [10, 11]. Studies have shown that during the healing process, the blastema tissue has cells with the ability of proliferation and differentiation, similar to embryonic cells that could differentiaton into different kinds of cells in vivo [12]. One of the best examples of blastema tissue formation in mammals is the replacement of all tissues after puncture of the pinna of the male New Zealand rabbit. Blastema consists of colonal shaped cells similar to mesenchymal cells which are formed on the injured sections. Blastema cells have totipotent characteristics and therefore are able to convert to different cells. Subsequently, they are formed in the margins of the injury, 2 - 3 days after punching which in turn result in the regeneration of holes on the pinna [13, 14].
2. Objectives
The purpose of the present study has been investigated to show the effects of New Zealand rabbit bladder as a scaffold on the blastema cells.
3. Materials and Methods
For this experimental study, 6 male New Zealand rabbits of 4 - 6 month of age and approximately 2 - 3 kg of weight were purchased from Razi Research Institute of Tehran and then transferred to the animal house of faculty of science, Islamic Azad University, Mashhad branch. Rabbits were kept in a 21°C room with a 12 hour light: dark cycle and were fed normal rabbit chow.
At all stages of this research, legislations of the ethics of working with laboratory animals including free access to food and water, painless killing, prevention of pain from punching such as the use of lidocaine were observed. To produce the decellularized scaffold of New Zealand rabbit bladder, at first rabbit bladder was isolated and divided into smaller equal pieces and transferred into a falcon tube. There are many factors for decellularization of tissues and organs which depend on density, lipid content, tissue thickness and rate of cells [15]. The chemical method was used for bladder decellularization. In this method all samples were put in a solution of 1% sodium dodecyle sulphate (SDS) for 24 hours. Then, they were washed with sterile distilled water and are sterilized with 75% ethanol and acetic acid and finally put in PBS (phosphate buffer saline) for 24 hours. After following this procedure, the scaffold of New Zealand rabbit bladder was ready to use [16, 17]. In the next stage the blastema ring was prepared. For this purpose, a rabbit was selected and put in a rabbit inhibitor to punch pinna and prepare blastema tissue. By using lidocaine spray all ear surfaces were completely anesthesized. Furthermore several holes of 2 mm diameter were made in the ears by using a special puncher. The rabbits were kept under rigidly sterile conditions for 3 days. At the end of the third day, a new hole was made on the previous hole with a puncher of 4 mm diameter and the blastema ring was obtained. Then, the blastema rings were completely sterilized in 7 stages, 6 stages of which involved placement in sterile normal saline and one stage of placement in culture medium. In this research, 500 mL DMEM (Dulbecco’s Modified Eagle’s Medium) and 100 mL FBS (fetal bovine serum) was used, which means each time of preparing the medium for consumption included 85 mL DMEM, 15 mL FBS and 500 mL penicillin streptomycin. Then, the scaffold of the prepared bladder was transferred to the laminar hood under sterile conditions and each New Zealand rabbit’s bladder scaffold was placed in the center of a blastema ring. After that, all samples were placed in a 6 house medium plate, after which the medium was added to them, and the plates were transferred to the CO2 incubator. On day 5, 10, 15, 20, 25, and 30 after removing them from the medium and histological stages, the samples were stained with Hematoxylin-Eosin and Peak Indigo and studied by light and Transitional Electron Microscopy.
4. Results
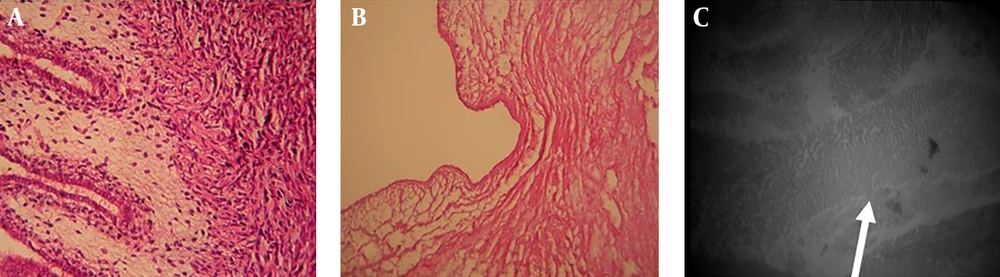
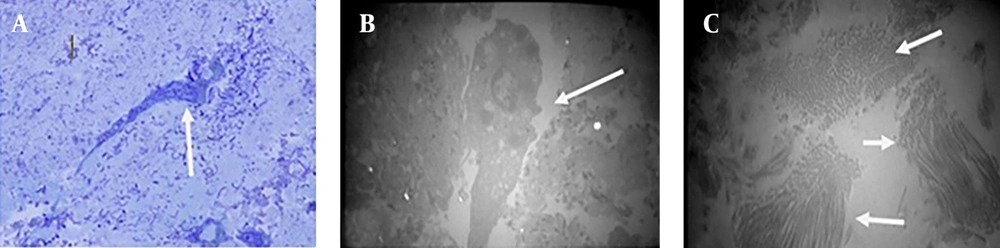
Comparing the decellularized New Zealand rabbit bladder with the control sample showed that the chemical method was suitable enough to produce scaffold by preserving extracellular matrix structure of the bladder (Figure 1 A, 1B). Further, study of transitional electron microscopy of the decellularized bladder showed that collagen fibers were also maintained in the scaffold (Figure 1 C).
4.1. Culture Results:
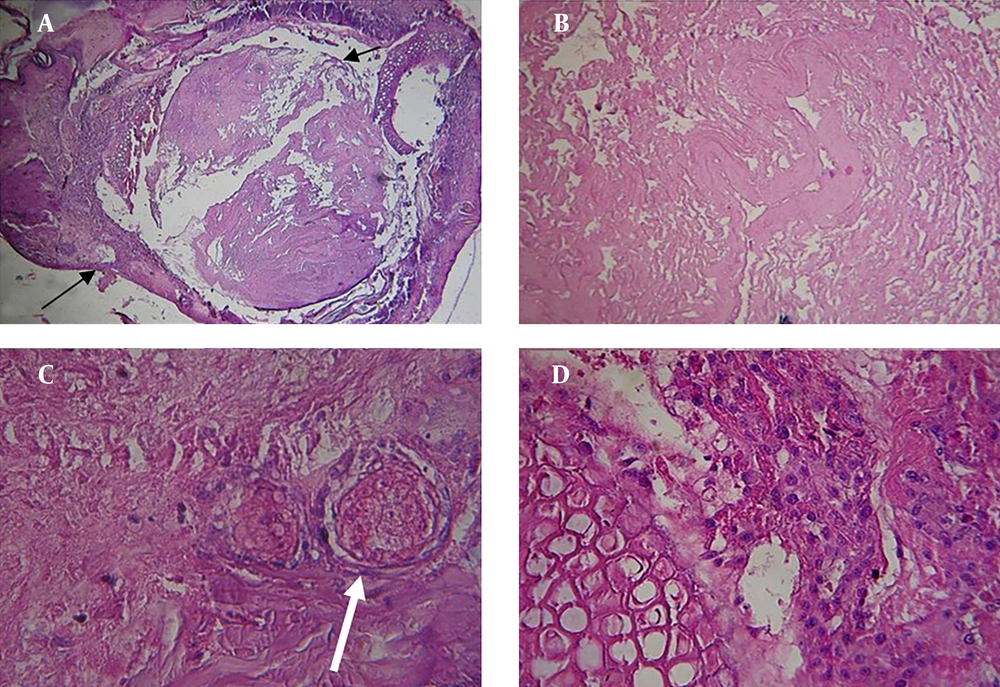
On the 5th culture day, no blastema cell migration had taken place from the blastema ring into the bladder scaffold and the blastema cells were seen only in the ring (Figure 2 A, 2B, 2C, 2D).
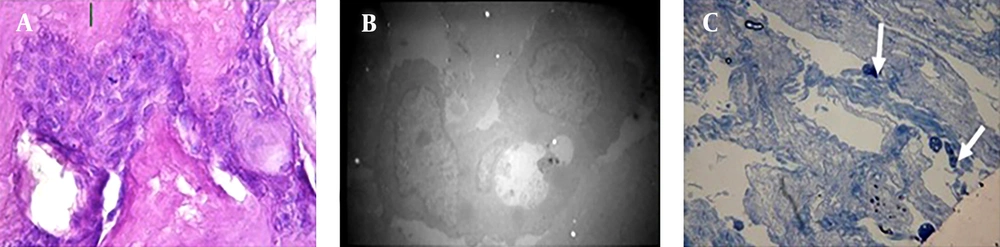
On the 10th culture day, blastema cells had migrated from the ring to the scaffold. The remarkable point was that the blastema cells which had migrated to the scaffold were connected together (Figure 3 A). Electron microscopy confirmed this fact (Figure 3 B). In Toluidine Blue staining sections a number of immature cells in the differentiation pathway of blastema to fibroblast cells, epithelial cells and probably chondrocytes were observed (Figure 3 C).
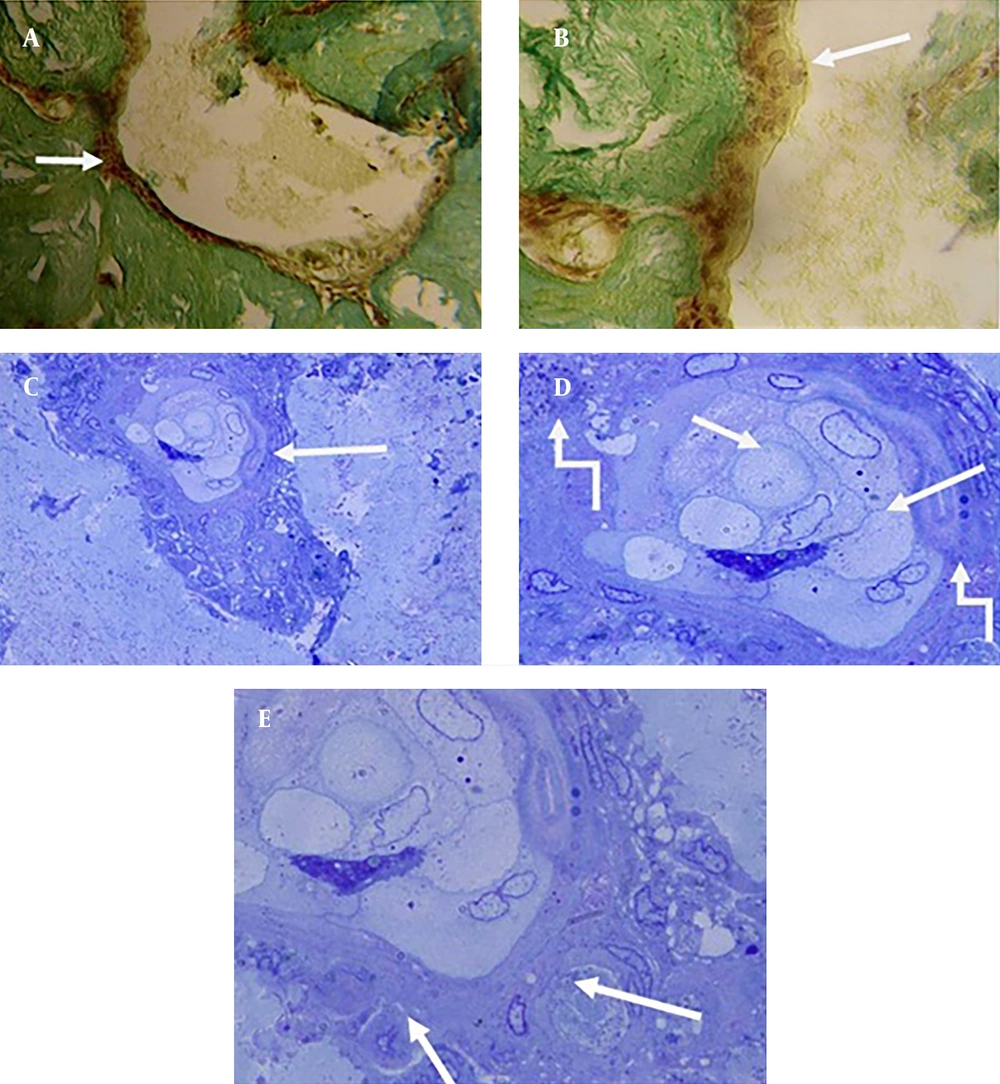
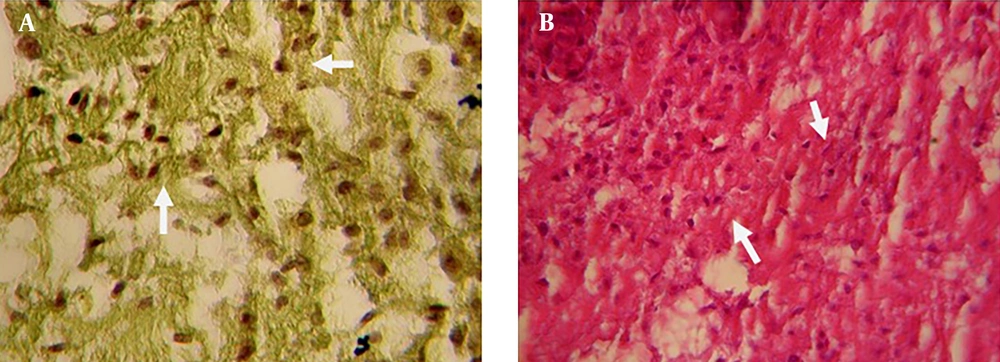
On the 15th culture day, Peak Indigo staining showed that migration of blastema cells to the scaffold was complete and differentiation had started. On the same day, the presence of cell colonies probably representing the formation of epithelial cells was observed (Figure 4 A, 4B).
With Toluidine Blue staining, desmosome junctions between epithelial cells were detected. The purple beads around the cell colonies showed metachromasy properties. The purple ribbon around the colonies was an indication of the presence of glycoproteins. These compounds were secreted from cells and made up the contents of the matrix (Figure 4 C, 4D). On the 20th culture day; in addition to epithelial cells, fibroblasts which were different from blastema cells were observed in the scaffold (Figure 5 A, 5B). Collagen fibers were also seen on the same day (Figure 5 C).
On the 25th and 30th days; cells that had migrated to the scaffold were scattered and not connected together. Thereby inactivation of cells could be seen and the cells had lost their normal shape (Figure 6 A, 6B, 6C). According to the result, bladder decellularized matrix could be a suitable scaffold to migration and differentiation of blastema cells.
A, B, Migration of blastema cells into the scaffold was complete, and differentiation into the epithelial cells had started (peak indigo stain 100 ×, 400 ×); C, 1 micron section of migrated blastema cells into the scaffold and differentiated to epithelial cells (toluidine blue, 200 ×); D, desmosome adhesion between epithelial cells, purple beads around the cells colonies had metachromasy characteristic (toluidine blue, 400 ×); E, the purple beads around the cell colonies was an indication of the presence of glycoproteins (400 ×).
5. Discussion
The results obtained on different days of culture revealed that migration of blastema cells from blastema ring into the bladder scaffold began 10 days after the culture. Most migration of blastema cells occurred on days 15 and 20 of culture. In addition, blastema cells were differentiated into epithelial and fibroblast cells.
Successfully engineered scaffold biomaterials which are capable of achieving regeneration must meet several design criteria. The scaffold must be conductive and possess physical properties with a three dimensional architecture and contain the necessary biological information to promote desired cellular processes. Some of these functions include attraction and interaction of appropriate cell populations, cell migration, differentiation etc. In this study we used decellularized New Zealand rabbit bladder as a scaffold to study effects of scaffold on the migration and differentiation of blastema cells. It was observed that progenitor cells from blastema tissue of rabbit pinna had expanded and differentiated into epithelial cells, fibroblast and probably chondrocyte cells. Moreover, the population of stem cells from blastema tissue had already been cultivated and explained as immortal cells expressing Oct4 and Sox2 markers [18] and also according to our findings, blastema cells tended to propagate in a colonogenic manner in the bladder scaffold. Colony formation is the other features described for MSCs [19, 20] which are a kind of adult stem cells are isolated from bone marrow [21, 22]. The Extracellular matrix can be considered as a morphogenetic code which is interpreted according to the cells that are in contact with it. Through the specific receptors on the cell surface, information of extracellular matrix can have a profound effect on cell behaviors including: adhesion, cell polarity, migration and signals that regulate cell survival, differentiation and proliferation [23]. According to the findings by the present study, it seems that bladder extracellular matrix components, cause induction and differentiation of blastema tissue-derived stem cells in vitro. Rozario and Desimone have already shown that extracellular matrix indirectly affected differentiation by regulating the cell shape and therefore affecting cell fate [24]. Baharara et al. showed the bovine bladder scaffold effect on blastema cells and their differentiation into fibroblast cells [25]. In addition, biologic scaffold materials composed of mammalian extracellular matrix are commonly used for the repair and reconstruction of injured tissues [26]. In the present study on the 15th day, probably blastema cells differentiated to epithelial and fibroblast cells. Experiments by Baghban-Eslaminejad and Bordbar have already shown that blastema cells differentiated to fibroblast cells in culture medium [13]. Based on our findings bladder scaffold affected blastema progenitor cells and differentiated to epithelial and fibroblast cells. Lindberg observed that fibroblasts usually invade the scaffold when cultured with epidermal cells on small intestinal sub mucosa (SIS) [27]. Some studies have shown blastema tissue-derived from stem cells are capable of differentiation in different aspects [28]. In this research New Zealand rabbit bladder was decellularized by chemical method using sodium dodecyl sulfate (SDS). According to Tian et al. experimental works, sodium dodecyl sulfate is a suitable material to produce scaffold in primary application of tissue engineering [29]. At last, one possibility would be that the cells themselves had no proper differentiation potential but the bladder extracellular matrix provided for them forced the cells to adapt to the corresponding differentiated phenotypes. The second possibility is that the cells possessed a true multipotent differentiation capacity.
It can be concluded that bladder scaffold has the ability to induce blastema tissue-derived stem cells as they are capable of differentiation and migration, and this event is considered very important because no growth factor or inducer component was used. Advances in the cell biology of extracellular matrix have provided new ways of thinking about the roles of matrix in development and pre-clinical applications.