1. Background
Sedentary life style is an independent risk factor for chronic diseases such as type 2 diabetes, hypertension, obesity, and cardiovascular diseases [1-4]. Physical inactivity leads to metabolic and structural changes among young and healthy individuals, such as muscle atrophy, increase in insulin resistance, inflammation, hypertension, and some undesirable changes in plasma lipid profile as well [5-8]. High blood pressure and impaired lipid profile are among the risk factors for cardiovascular diseases. They are also considered as components of metabolic syndrome [9]. Pharmacological interventions in order to prevent and treat cardiovascular diseases and control the circulating cholesterol levels have increased in recent decades. Considering the fact that these medicines are chemically produced, they usually have side effects which may lead to further complications such as digestive problems. Modifying the lifestyle through regular exercise and appropriate nutrition can prevent subsequent hypertension and impaired lipid profile [10]. Evidence from several studies supports an inverse relation between regular physical activity and the rate of CVDs (cardiovascular diseases) [11, 12]. Furthermore, regular exercise, particularly aerobic exercise, has the capacity to ameliorate several cardiovascular risk factors. Much evidence suggest that regular exercise may improve several risk factors for CVD, including lowering blood pressure, favorably altering blood lipid profiles, and improving blood vessel function [13-15]. Haskell reported that moderate-intensity, endurance-type activity produce favorable lipoprotein changes in healthy persons as well as in patients with ischemic heart disease, diabetes, and renal failure. They suggested that these exercise effects are most likely mediated by alterations in the activity of enzymes involved in the synthesis, transport and catabolism of the various lipoproteins including lipoprotein lipase, hepatic lipase and lecithin cholesterol acyltransferase [16].
On the other hand, it is indicated that garlic has a favorable effect on lipid profile and blood pressure [17, 18]. The blood pressure lowering effect of garlic is one of its 1st known effects, which was confirmed later in animal and human studies [18, 19]. Ried et al. in a review indicated that a regular consumption of garlic decreases the blood pressure and this effect is even more in hypertensive subjects [20]. Moreover, it is shown that garlic decreases the intestinal absorption of cholesterol and modulates cholesterol synthesis [17, 21, 22]. Powolny and Singh indicated that garlic powder consumption (400 mg/day) for 4 weeks decreases serum levels of total cholesterol, triglyceride and LDL-C [23]. On the contrary, van Doorn et al. reported that daily consumption of garlic powder (2.1 g/day) for 1 and 3 months had no significant effect on lipid profile in individuals who were prone to cardiovascular disease [24].
Seo et al. investigated the effect of regular exercise and aged garlic extract (AGE) in combination among postmenopausal women. They suggested that AGE supplementation reduced cardiovascular risk factors independently of exercise in postmenopausal women [25]. Therefore, the effect of combined intervention is largely unknown. Considering the literature, combining intervention may be more effective than either intervention alone. In the present study, we made a placebo-controlled comparison of the effects of garlic supplementation and regular aerobic exercise in combination, on blood pressure and lipid profile in inactive young men.
2. Objectives
We aimed to investigate the effect of aerobic training along with garlic supplementation on lipid profile and blood pressure in inactive young men.
3. Patients and Methods
Forty-four young, healthy, inactive men (20 to 30 years) participated in this semi-experimental study (Table 1). The sample was generally representative of the overall population of the men students of Tabriz Islamic Azad University. After giving a complete description of issue, aims, and methods, the volunteers filled the consent form and general health check list. The experimental procedures and the study protocols were approved by the Ethical Committee of Tabriz University of Medical Sciences, Iran. None of the individuals had a history of smoking, diseases, taking special medicines, and regular sport activities, at least for 2 years. Initial suitability for the study was determined by completion of a simple lifestyle questionnaire. Subjects were excluded if they exercised > 1 time/week for the purposes of improving their health. At the beginning and after the experimental period, the anthropometric characteristics (height, weight, body mass index and waist/hip ratio) were measured. Body mass index was calculated through its formula (Weight (kg)/ Height 2 (m)). The skin folds were obtained using Harpenden skin fold caliper on the right side of the body at the following sites: chest, abdominal, and thigh. Body fat percent was estimated through Jackson Pollock formula [26]. Subjects were randomly divided into 4 groups including: 1) Training + Garlic (TG), 2) Garlic (G), 3) Training + Placebo (TP) and 4) Placebo (P). It should be mentioned that some of the subjects were omitted from the groups because of personal reasons, inconsistency in performing exercise trainings and irregular use of supplement or placebo and eventually, nine final subjects remained in each group. A double-blind randomized placebo-controlled trial was performed over a four-week period. Subjects in the TG and G groups received one garlic capsule (500 mg Allicin) 2 times per day over a period of 4 weeks. In addition to garlic, subjects in the TG group performed a progressive aerobic program. Subjects in the TP group performed exercise training with no garlic supplementation, while the subjects in the P group received one capsule (500 mg starch) 2 times per day as the placebo with no exercise training.
The intensity and duration of aerobic training were 60% of HR max and 30 minutes for the first week, 65% of HR max and 30 minutes for second week, 70% HR max and 45 minutes for third week and 75% of HR max and 45 minutes for the fourth week. The exercise training program consisted of running on treadmill 4 times/week. It should be mentioned that the training protocol was prescribed based on ACSM (American College of Sports Medicine) suggestions [27]. All individuals were asked to follow their usual diet and avoid taking any supplements during the study. Moreover, before taking primary and final blood samples, the 24-hour diet recall questionnaire (reliability the validity of the applied questionnaire was r = 0.078) was applied in order to control the subject's diet the day before taking blood sample [28]. Commercial garlic tablets (nature made, US) were used in this study. Garlic tablets were powdered and encapsulated in gelatin capsules. Each capsule contained 500 mg of Allicin or 500 mg of starch as placebo. The aspect of garlic and placebo capsules was absolutely identical. The subjects were instructed to consume 1 capsule 2 times/day after breakfast and dinner. They were also instructed to avoid consumption of any other supplements during the entire study period. Before and 48 hours after the experimental period, all subjects were required to avoid eating and drinking after midnight and attend the lab in a fasting state. At these sessions, after thirty minutes resting in a sitting position blood pressure was measured and their blood samples were taken from antecubital vein for lipid profile analysis. Triglycerides, HDL-cholesterol, total cholesterol were assessed using enzymatic kits (Pars Azmoon, Iran). LDL-cholesterol was calculated through Friedwald formula [29]. The data are expressed as Means ± Standard Deviations. Distribution of data was assessed for normality using Kolmogorov-Smirnov test. Both intragroup and intergroup comparisons were made using the 2-way repeated measures analysis of variance followed by the Bonferroni post-hoc test. All statistical analyses were performed using the SPSS 18 statistical software package. Statistical significance was set at P < 0.05.
| Variable | Training + Placebo | Garlic | Training + Garlic | Placebo | ||||
|---|---|---|---|---|---|---|---|---|
| 21.88 ± 1.53 | 23.22 ± 2.04 | 23.00 ± 1.00 | 23.55 ± 1.50 | |||||
| 179.39 ± 6.09 | 177.22 ± 8.17 | 175.94 ± 4.86 | 176.22 ± 3.57 | |||||
| 71.73 ± 9.30 | 71.67 ± 8.99 | 69.25 ± 9.10 | 69.37 ± 8.93 | 71.77 ± 8.30 | 71.54 ± 8.04 | 66.85 ± 6.74 | 67.03 ± 6.60 | |
| 22.26 ± 2.51 | 22.24 ± 2.42 | 22.07 ± 2.74 | 22.08 ± 2.71 | 23.15 ± 2.15 | 23.07 ± 2.09 | 21.51 ± 1.79 | 21.57 ± 1.85 | |
| 16.53 ± 4.47 | 15.96 ± 3.94 | 14.95 ± 5.15 | 14.88 ± 5.13 | 14.30 ± 4.95 | 14.15 ± 4.92 | 13.72 ± 5.20 | 14.20 ± 5.12 | |
| 117.33 ± 44.41 | 113.33 ± 27.10 | 124.78 ± 73.57 | 114.4 ± 47.39 | 113.22 ± 43.00 | 104.78 ± 44.11 | 110.11 ± 43.71 | 109.33 ± 41.55 | |
| 155.11 ± 18.05 | 150.44 ± 21.26 | 165.22 ± 22.22 | 156.11 ± 25.97 | 164.33 ± 20.30 | 156.22 ± 22.59 | 158.44 ± 17.04 | 160.89 ± 16.82 | |
| 67.11±8.07 | 61.00±15.79 | 71.96±18.31 | 64.29±20.69 | 75.03±21.10 | 66.18±23.20 | 67.51±6.49 | 70.44±13.72 | |
| 48.88 ± 8.37 | 51.66 ± 7.84 | 51.66 ± 3.50 | 54.66 ± 8.13 | 51.55 ± 11.14 | 55.11 ± 8.90 | 54.22 ± 8.36 | 54.00 ± 7.38 | |
| 124.67 ± 8.30 | 118.1 ± 8.32 | 126.20 ± 6.83 | 123.78 ± 7.46 | 127.00 ± 8.66 | 119.89 ± 5.27 | 121.00 ± 7.85 | 122.67 ± 6.46 | |
| 76.5 ± 7.29 | 73.22 ± 6.8 | 79.88 ± 8.47 | 72.77 ± 7.96 | 76.77 ± 5.06 | 68.77 ± 6.49 | 76.66 ± 7.5 | 75.44 ± 6.78 | |
4. Results
The baseline (week 0) characteristics of the subjects are shown in Table 1. As it is presented in Table 1, all groups were of similar age. Body weight and composition did not differ between the 4 groups before or after the study, nor were there significant changes during the study.
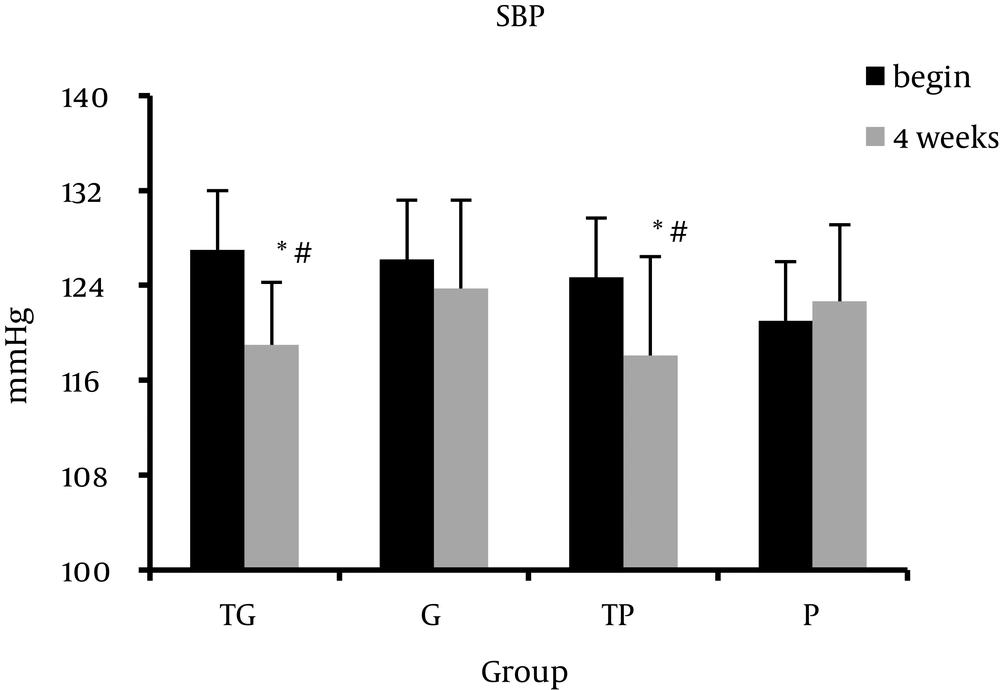
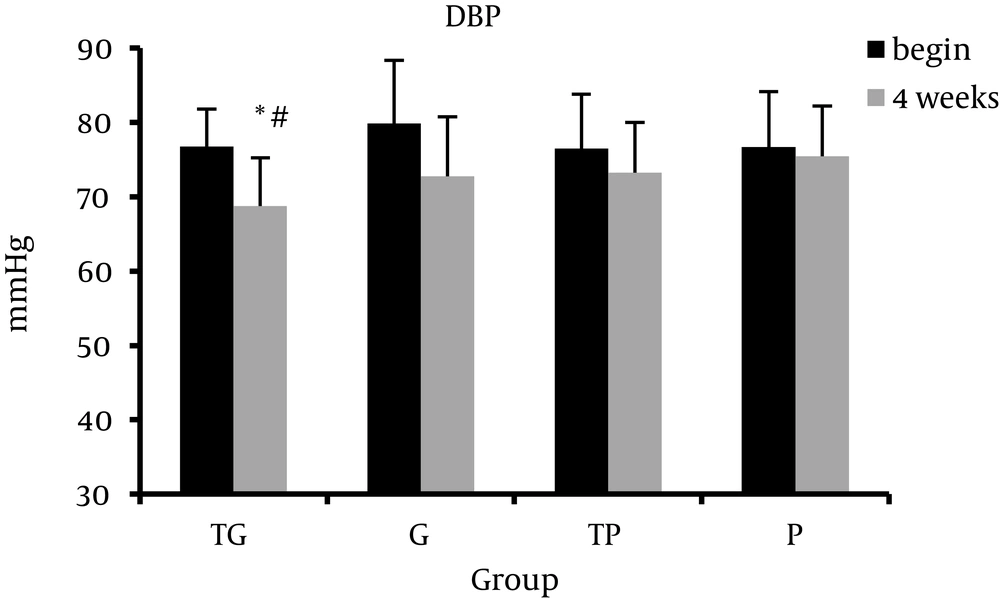
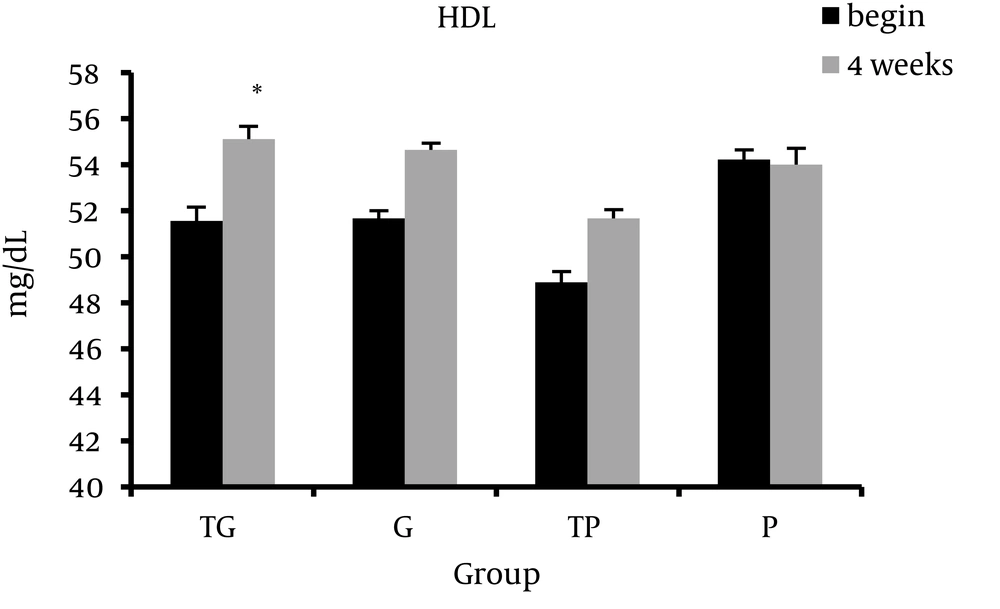
Table 1 presents the Mean ± Standard Deviation of the body composition, lipid profile, systolic blood pressure, and diastolic blood pressure at baseline (week 0) and after 4 weeks of intervention (week 4). ANOVA analysis showed that prior to study there were no significant groups differences in any variables. Intragroup analysis showed that systolic blood pressure and diastolic blood pressure significantly decreased in TG group. Furthermore, data analysis showed a significant intergroup difference. Post hoc analysis showed a significant difference between TG and P groups in systolic blood pressure (SBP) and diastolic blood pressure (DBP) at week 4 (P = 0.023, P = 0.038, respectively). After 4 week of intervention, the TP group exhibited significant decrease in systolic blood pressure (P = 0.01). There was also a significant difference in systolic blood pressure between TP and P groups at week 4 (P = 0.022). We observed no significant alterations in lipid profile indices in each group except HDL-C levels in TG group (P > 0.05). HDL-C levels increased in TG group at week 4 compared to baseline (week 0) (P = 0.038) (Figures 1-3).
5. Discussion
The results indicated that garlic supplementation combined with exercise training improved blood pressure but was not effective on lipid profile in inactive subjects. Both regular exercise and garlic have the potential to improve lipid profile and blood pressure in healthy and patient subjects [8, 19, 30, 31]. These findings are consistent with a number of previous studies of long-term exercise. Previous studies have shown a negative relation between blood pressure and regular physical activities [32]. van Hoof et al. indicated that after 4 months of regular aerobic exercise, diastolic blood pressure was significantly reduced among inactive individuals but the changes in systolic blood pressure was not significant [33]. In consistent with our study, Wilmore et al. reported that 20 weeks of regular aerobic activity significantly decreased systolic and diastolic blood pressure among inactive individuals [34]. Several mechanisms have been suggested to the blood pressure lowering effect of regular exercise. Decrease in the activity of the autonomic nervous system and following reduction in systemic vascular resistance is most likely involved in the exercise-induced reduction of blood pressure [31]. Moreover, the rennin-angiotensin system might be involved. Decrease in the activity of the sympathetic nervous system caused by regular exercise also affects the kidney, which is the most potent factor in long-term blood pressure regulation [35]. Improvement in endothelial function is another potentially important mechanism. Higashi et al. reported that long-term mild physical exercise improves endothelium-dependent vasorelaxation and lowers blood pressure in patients with mild essential hypertension [36]. On the other hand, several studies have been performed on the effect of garlic consumption on blood pressure in healthy and patient individuals. Ried et al. showed that garlic consumption reduces the blood pressure, especially in hypertensive individuals [20]. Beneficial effects of garlic have been attributed to the sulfur containing compounds present in garlic. Researchers have postulated that antihypertensive action of garlic is due to its prostaglandin like effects, which decreases peripheral vascular resistance [19]. It is shown that gamma-glutamylcysteine compounds in garlic inhibits angiotensin-converting enzyme in vitro which might be involved in blood pressure lowering effect of garlic [37]. Garlic modulates the production and function of both endothelium derived relaxing and constricting factors. A number of studies have indicated that garlic elicits nitric-oxide-dependent relaxation in pulmonary arteries [38, 39], but another study reported that vasodilatory effect of allicin is independent of the synthesis of nitric oxide [40]. Methyl allyl tri-sulfide derived from allicin decreases the blood pressure through its vasodilatory effects [41]. In the present study garlic supplementation alone had no significant effect on blood pressure, but in combination with aerobic training was more effective.
Besides blood pressure lowering effect, garlic has favorable effect on lipid profile. It has been suggested that garlic attenuates the hepatic activities of lipogenic and cholesterogenic enzymes [22], increases the excretion of cholesterol [42] and inhibits cholesterol synthesis [22, 43]. In the present study we observed no significant intergroup differences in lipid profile which indicated garlic supplementation combined with exercise training had no additive effect on lipid profile. Inconsistent with our study, Seo et al. reported that Aged Garlic Extract (AGE) combined with regular exercise (aerobic and resistance) reduced cardiovascular risk factors in postmenopausal women but the changes were independent of exercise [25]. Previous studies have suggested several mechanisms by which regular exercise affects lipid profile [44]. A possible mechanism modulating the HDL-C is the pathway of reverse cholesterol [45]. This pathway removes cholesterol from the circulation and distributes it to the liver where it is processed for excretion [46]. Furthermore, it is well documented that regular exercise increases the lipoprotein lipase (LPL) gene expression and activity in skeletal muscle resulting in decreased plasma triglyceride content [47]. In addition, decrease in LDL-C levels could be attributed to the reduction in the activity of hepatic triglyceride lipase enzyme during long-term physical exercise [48]. A few studies have also suggested that the HDL-raising effect of aerobic training could be largely explained by the concomitant loss of body mass or fat [49]. We observed no significant change in body composition after four weeks of aerobic exercise. The lack of exercise effect on lipid profile in the present study might have been attributable to the lack of weight loss during the experimental period. Furthermore, a limitation for the present study was the experimental period. A longer study period may yield different results. Based on the findings of the present study, it is concluded that garlic supplementation along with exercise training might have additive effect on blood pressure but not lipid profile. Future research should investigate the efficacy of this combined intervention over a longer duration. Moreover, further studies are needed to determine the effect of this combined intervention in other populations such as hypercholesterolemic and hypertensive patients.