1. Background
Cervical cancer is one of the leading causes of death in women worldwide and its global incidence increased at an annual rate of 0.6% between 1980 and 2010 [1]. Although cervical cancer mortality rates have been decreasing, the recurrence and metastasis of cervical cancer to other parts such as the lymph nodes [2, 3], lungs [4, 5], bones [6, 7], liver [8] and bowels [9] are main factors contributing to mortality in patients with cervical carcinoma. Thus, apart from surgery and the destruction of cervical cancer cells by medications, inhibiting metastasis is an auxiliary strategy for treating patients with cancers. Cancer metastasis leads to poor clinical outcomes and mortality in patients with cancers. Metastasis process involves cell adhesion, migration, invasion and proteolytic degradation of extracellular matrix (ECM) [10]. During the invasion and metastasis, cancer cells move within tissues, thus the control of migration could be critical key in treatment of metastatic cancer [11]. Reorganization of the actin cytoskeleton is the primary mechanism of cell motility and it is essential for most types of cell migration. In the metastatic process, the actin cytoskeleton and its regulatory proteins are necessary for cancer cell migration. According to results, the ability of Rho GTPase family members has been proved in the regulation of cell movement, cell adhesion and migration that this could be an important role in the invasion and metastasis of cancer cells [12]. Rac1, small protein of Rho GTPase family, performed multiple cellular activities on actin. This protein plays an important role in degradation of adhesive joints cell- cell, resistance to apoptosis and cell proliferation. Thus, Rac1 accelerates the invasion of cancer cells [13, 14]. Therefore, study on this protein is important for investigating molecular mechanisms of cancer cells metastasis. Herbal medicines have been used for treatment a variety of cancers including leukemia, cervical, ovarian, testicular, lung, liver, esophageal, stomach, colon, and rectum cancer [15]. Flavonoids possess anticancer and chemopreventive properties through their antioxidant activity and evidences indicate that some flavonoids are potent chemopreventive agents with low cytotoxicity [16-18]. Flavonoids or phenolic acids are plant pigments and soluble in water. This group is responsible for the antioxidant capacity of fruits and vegetables [19]. Over 4000 different flavonoids have been identified in the major groups of flavonoids [20]. Quercetin (3, 3, 4, 5, 7-pantahydroxyflavone), a flavone-3ol-class of flavonoid, is ubiquitously present in apples, onions and other vegetables [21]. Quercetin is without carbohydrate [22, 23] and has been the most studied flavonoids to determine the biological effects of this component [20].
This compound has a wide range of medical, pharmaceutical and biological applications [24]. Quercetin exhibits a variety of biological functions, including anti-oxidative, anti-inflammatory, anti-cancer and anti-metastatic activities. Studies have shown that quercetin can inhibit the proliferation of a wide range of cancers, including colon, cervix, lung, breast and prostate [25, 26]. This polyphenolic compound has been reported to modulate signal transduction pathways associated with cell proliferation and differentiation, apoptosis, angiogenesis and metastasis [27, 28].
2. Objectives
According to the role of Rac1 in the migration and invasion of cancer cells and also the importance of cervical cancer, therefore, in present study we investigated anticancer effects of quercetin through its effects on metastasis based on the amount of Rac1 expression in cervical cancer cells.
3. Materials and Methods
In this experimental study, quercetin, penicillin, streptomycin and DMEM culture medium were purchased from GIBCO BRL (Grand Island NY, USA). Trypsin and fetal bovine serum (FBS) were obtained from GIBCO. Primary antibody anti-Rac1 from cell signaling (cut number: 2465s), Goat-anti rabbit IgG-HRP secondary antibody from Sigma and ECL kit were obtained from Isfahan CMG. Other solutions and reagents were from the Merck Company in this research.
3.1. Cell Culture
This study was performed in Yazd University of Medical Sciences. HeLa cells were obtained from the Pasteur Institute of Tehran, Iran. Cells were grown in Dulbeccos modified Eagles medium (DMEM) containing 10% fetal bovine serum, 0.30% sodium bicarbonate and antibiotics (streptomycin 100 µg/mL and penicillin 100 IU/mg) cells were maintained in a humidified atmosphere of 5% CO2 at 37°C and subcultured every 3 or 4 days with 0.05% trypsin (Figure 1). Then cells were treated with 20 and 40 µM concentration of stock solution of quercetin for 24 hours. These concentrations were according to IC50 = 80 µM of quercetin on cervical cancer cells [29], the cells are maximum viability in the concentrations lower than 80 µM. Moreover, we have control sample that all conditions were similar to test groups but without quercetin.
3.2. Preparation of Stock Solution Quercetin
Stock solution of quercetin (100 mM) prepared by dissolving 0.0151 grams quercetin powder in 0.5% DMSO was diluted with Dulbecco’s modified Eagles medium (DMEM) prior to use to obtain the desired concentration (20 and 40 µM). The final concentration of DMSO used for treatment was 0.1% (v/v).
3.3. Electrophoresis and Westernblotting
After 24 hours of treatment, cells washed with ice phosphate-buffered saline (PBS) and lysed in a lysis buffer [50 mM Tris-HCl (pH = 7.4), 1% Nonidet P-40, 40 mM NaF, 10 mM NaCl, 10 mM Na3VO4, 1 mM phenylmethanesulfony, l fL uoride (PMSF) and 10 mM dithiothreitol (DTT)]. The cell lysates were centrifuged at 12,000 rpm for 5 minutes. Total protein of supernatant was determined by the method of Bradford [30]. A total of 60 µg of this suspension were applied to 10% SDS-polyacrylamide electrophoresis gels (PAGEs) at 100 V for 1 hour. The separated proteins were then electrophoretically transferred to nitrocellulose overnight at 30 V at 4°C. The membranes were incubated in blocking solution containing 5% non-fat dry milk and Tris-buffered saline/0.1% Tween-20 for 2 hours to block non-specific binding sites. After 2 hours blocking, blots washed in Tris-buffered saline/0.1% Tween-20 (TBST) four times, then the blots were incubated overnight at 4°C with 1:1000 dilutions of primary antibody. After 24 hours, washed in TBST buffer (4 times) and probed for 1 hour with 1:5000 dilutions HRP-conjugated secondary antibody at room temperature. After washing with TBST buffer (4 times), the immunoreactive proteins were visualized using enhanced chemiluminescence (ECL) detection reagents. Protein expression levels were calculated by the program Gene Tools Gel Document sets and were reported as relative percentage. For western blotting analysis, acting used as internal standard. Also for quantifying of target proteins, resulting film scanning densitometry were determined relative to acting levels. The experiment was replicated three different times.
3.4. Statistical Analysis
The results analyzed using SPSS-16. All values were expressed as the mean ± standard error of mean (SEM). Data were analyzed by repeated measures analysis of variance (ANOVA) followed by post-hoc test for determine the significance of differences between treatment groups. Statistical significance was accepted for P < 0.05.
4. Results
In order to evaluate effect of quercetin on Rac1 expression levels, 20 and 40 µM concentrations of quercetin were used for HeLa cells since these concentrations and time are the best sources to evaluate effect of quercetin on these cells [29].
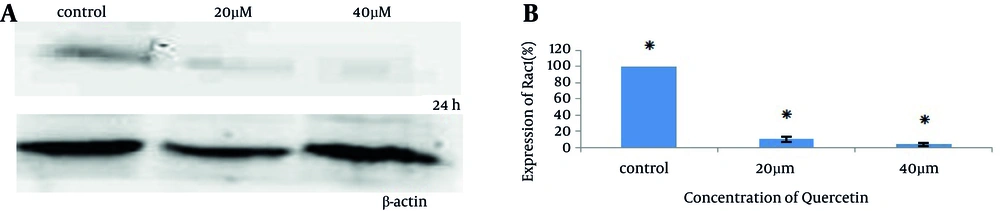
Analysis of Rac1 by western blotting revealed a significant reduction in Rac1 expression (21 kD) in cells treated with quercetin compared to untreated control (Figure 2). Figure 2 A showed that the expression levels of Rac1 decreased in a dose-dependent manner. Rac1 expression was obtained 100 ± 0, 10.4 ± 3.14, and 3.59 ± 2.4 in control group, 20 µM and 40 µM quercetin, respectively.
5. Discussion
In the present study, we evaluated the mechanism of antimetastatic activity quercetin. The majority cancer deaths occur as an outcome of metastasis rather than the original tumor; therefore, inhibiting cancer-cell metastasis is an important aspect of cancer prevention. With increasing application of plant derived cancer chemotherapeutic agents probing the antiproliferative and antimetastatic effects of phytochemicals have gained enhancing momentum for anticancer drug design [29]. This study showed that the quercetin inhibited Rac1 expression as a marker of metastasis in cervical cancer cells that it can be shown that quercetin may have potential antimetastatic effects on cancer cells. Amount of the inhibitory becomes more by increasing concentrations of quercetin. A study was carried out on HeLa cells showed that quercetin could inhibit adhesion and migration and invasion this cells. Quercetin could enhance the inhibitory effect of cis-platin on HeLa cell adhesion, migration and invasion but the exact mechanism was not understood [31]. The polyphenols in green tea, including catechin derivatives, suppressed the invasive behavior of MDA-MB231 cells and the antiinvasive effect occurred. Other study were performed on breast cancer cells MCF-7, shown that EGCG blocked the adhesion of MCF-7 cells to ECM, fibronection and vitronectin and so suppressed migration and invasion of these cells. These effects consequenced from the inhibition of NFkB, VEGF, MMP-2 integrin receptor-α, β, VASP, FAK and Rac1 by EGCG [32]. Liu et al. investigated the Rac1 signaling pathway on liver cancer (HCC) in vivo and in vitro conditions. In this study it was revealed the anti-metastatic effect of melitin on the cancer cells via rac1 suppression. Expression of Rac1 was measured by using Western blotting technique in a variety of liver cancer cell lines and it was concluded that Rac1 expression in HCC cells would increase invasive and it directly would relate to metastasis of this cells [33]. In 2004, the protein expression of Rho, Rac1 and cdc42 in tumor samples from surgical specimens of 57 patients with tumors of the testis (Testicular cancer) and non-tumoric were measured by using Western blotting techniques. The results indicated that the expression of these proteins in tumor samples was significantly higher than non-tumoric samples, and it was also reported that expression of Rho, Rac1 and cdc42 proteins in advanced stages of cancer is higher than in the early stages of cancer [34]. Expression of Rac1 and PAK proteins, a potent effector of Rac1, was evaluated on four groups including tumor and non-tumoric tissue, metastatic and normal lymph nodes in the upper urinary tract cancer and the results showed that expression levels of active Rac1 and PAK in tumor and metastatic lymph nodes were significantly higher than their levels in samples non-tumoric [35]. Considering that active Rac1 can mediate looseness of adhesion connections and subsequently accelerate migratory phenotype [13, 14].
Based on this study, we speculated that quercetin may affect the Rac1 pathway, which has been reported to be critical for inhibiting migration and invasion in tumor cells. Quercetin is a nontoxic and non-allergic dietary flavonoid that has been shown to possess antimetastatic properties. Consequently, consumption of natural compounds is very important to increase efficiency of cancer treatment. Results from the present study and previous studies beneficial effects of the quercetin flavonoid on migration and cancer progression was showed, therefore, the use of flavonoids as treatment with other pharmacological agents was suggested but the exact further studies on cellular and animal models were recommended. In summary, we have demonstrated that treatment with quercetin inhibits expression of Rac1 protein in HeLa cells. This is the first study to demonstrate that quercetin might be a novel anticancer agent for the treatment of cervical cancer through inhibiting migration and invasion. These results displayed that Rac1 inhibition may apply a profound influence on quercetin-mediated inhibition of tumor metastasis. Future studies will focus on elucidating the role of Rac1 in quercetin-mediated signaling.