1. Background
The heart has a very high energy demand and to sustain sufficient ATP generation, can use a variety of different carbon substrates as energy sources if available [1]. Fatty acids are an important fuel source for heart [2]. During exercise, the healthy heart can increase myocardial oxygen consumption four to six fold above resting values oxidize more fatty acids, which could reduce circulating fatty acids [1].
The exercise has been shown to increase genes which are involved in fat metabolism. The responses of ATP-binding cassette protein transporters A and G families members, particularly, ABCA1 and ABCG1 to different types of physical exercise in human and animal has also been investigated by several researchers [3-6]. ABCA1 is a multispan molecule with high expression level in the heart and other tissues and up-regulated by several factors such as cholesterol influx and physical activity [5]. ABCG1 is also expressed in numerous tissues including the heart, and transports phospholipids and cholesterol [6].
On the other hand, the role of some of the known nuclear receptors such as liver X receptor (LXR) and peroxisome proliferator activated receptor (PPARs) in ABCA1 and ABCG1 regulation under normal, disease, diet and physical exercise have been studied by several investigators [7, 8]. PPAR-α is thought to be the primary transcriptional regulator of fat metabolism in tissues with high fatty acid oxidation rates, particularly in heart [1] and LXR was identified as the nuclear receptor target of the cholesterol metabolites, oxysterols and able to lower cholesterol levels [9].
The role of various medicinal plants in reducing heart disease is well established. Crataegus species is well known in phytotherapy for the treatment of many cardiovascular diseases [10]. Carategus pentaegyna (Sorkh valik) is the one of best known and favorite edible wild fruit of the Crataegus species, (Rosaceae) which is exist in Mazandaran and other Northern states of Iran [11]. This fruit is believed to have some medicinal beneficial effect on cardiovascular function, blood pressure and lipid metabolism [12]. In Mazandaran province, this fruit is consumed freshly and as jam, vinegar and sauce. With consider to the above mention properties for Sorkh valik, there is very less information about the effect of the Sorkh valik extraction on gene expression. However, the response of the selected genes; such as LXR, PPAR-α, ABCA1 and ABCG1 to the Sorkh valik extraction alone or with an intensive treadmill running in rat heart did not take in attention and consideration.
2. Objectives
The purpose of the current study was to investigate the effect of a 8 weeks intensive treadmill running program on ABCA1, ABCG1, PPAR-α and LXR genes expression in male rat heart. The other aim of this study was to see the supplementation of Sorkh valik fruit extraction for 6 weeks could reinforce the possible effect of exercise on ABCA1, ABCG1, PPAR-α and LXR genes expression.
3. Materials and Methods
In this experimental study, all experiments involving animals were conducted according to the policy of Iranian convention for the protection of vertebrate animals used for experimental and other scientific purposes; the protocol was approved by the Ethics Committee of the Sciences, University of Mazandaran and Babol University of Medical Sciences.
Twenty Wistar male rats (4-6 weeks old, 158.9 ± 9.59 g weight) were acquired from the Pasteur's Institute (Amol, Mazandaran) and maintained in the Central Animal House of the Faculty of Physical Education and Sports Sciences of University of Mazandaran. Five rats were housed per cage with a 12:12 h light-dark cycle. Temperature and humidity were maintained at 22 ± 1.4°C and 50 ± 5%, respectively. Diets (a pellet form) and water were provided ad libitum. Animals were randomly assigned into control (n = 10) and training (n = 10) groups. Rats were further divided into saline-control (SC, n = 5), saline-training (ST, n = 5), Sorkh valik-control (SVC, n = 5), and Sorkh valik-training (SVT, n = 5). The control groups remained sedentary; whereas the training groups underwent a high intensity (34 m/min) treadmill running program. The animals were familiarized with animal room, treadmill apparatus for 10 days. The exercise groups were trained on a motor driven treadmill for 8 weeks according to the previously reported elsewhere [3]. The rats were submitted to run at low intensity [15 m/min, for 20 min] and then speeds and duration gradually were increased and at the first of third week rats were able to run at 34 m/min for 60 minutes, 5 days/week [13]. In this point, rats were run at a fixed intensity and duration for next 6 weeks. The animals were killed 72 hours after the last exercise session. Food but not water was removed from the cages 3 h before the sacrifices.
The ripped fruit samples of red Sorkh valik (Sorkh valik) were collected from the Neka forest (Mid Autumn, 2012) in the Mazandaran province of Iran. Fruits were dried in oven at 35ºC for 4 days and fine powdered by using a plant material was identified by herbarium collection in Department of Biology, Faculty of Basic Sciences, University of Mazandaran. The whole ripped and dried fruits of Sorkh valik were grounded in house electronic grinder to a fine powder. The extract was prepared according to the Cai et al. [14]. Rats were orally received a single dose (500 mg/kg of body weight or 10 mL/kg of body weight) of liquid extraction of Sorkh valik at the end of daily exercise training session [15]. The saline groups were treated with the same volume of saline solution. The fine powdered of Sorkh valik whole fruit which macerated by n-hexane (Merck Co., USA) for 72 hours at room temperature, extracted by soxhlet and evaporated using a rotary evaporator. Chromatographic analysis was carried out on HP devices, 6890 series GCMS apparatus combined with a mass selective detector. The capillary column used was HP-1MS. Helium was used as carrier gas. The components of Sorkh valik extracts were determined using library search soft-ware from Wiley/NBS Registry Mass Spectral Data and in-house “BASER Library of Fatty Acid Constituents.” Seventy-two hours after the last training session, rats were anesthetized with intra peritoneal administration of a mixture of ketamine (30-50 mg/kg) and xylazine (3-5 mg/kg). The heart was excised, cleaned, divided into two pieces, washed in ice-cold saline and immediately stored at -70ºC until RNA extraction. Total RNA was extracted from 30 mg of tissue using RNA purification kits (QIAGEN, Cat. No: 74104, Germany). Complementary DNA (cDNA) was extended by using cDNA synthesis kit [Quanti Tect Reverse Transcription Kit cDNA synthesis (Qiagen), (Cat. No: 205310, Germany)] according to the manufacturer’s instructions. Real-time quantitative PCR was performed using Quanti Fast SYBR Green PCR Kit (Cat. No. 204052; Qiagen, GmbH, Germany) in using 10 µL reaction containing 1 µL single-strand cDNA, 5 µL Master Mix, 1 µL of the each forward and reverse primers and 2 µL RNase-free water. Expected fragment size and oligonucleotide primer sequences for all selected genes are listed in Table 1. The PCR was carried out on BIO-RAD [C1000 TM Thermal Cycler] real time PCR system. The parameters for a two-step PCR were 95ºC, for 5 min, 1 cycle, then 95ºC, for 10 seconds and then 60ºC, for 30 seconds, 45 cycles and parameters for melting curve program were 55ºC to 95ºC (increment 0.5ºC for 5 seconds). Product specificity was confirmed in the initial experiments by 1.5% agarose gel electrophoresis and routinely by melting curve analysis. For statistical analysis, first, the relative levels of mRNA were analyzed by using a comparative threshold cycle method [16]. The Kolmogorov-Smirnov test was used to determine the normality of distribution, and variables were found to be normally distributed. A two way ANOVA were employed and significance was accepted at P < 0.05. All findings were expressed as means ± SEM and SPSS-13 (Chicago, IL) software was used for data analysis.
| Gene | Forward and Reverse Primer Sequences | Annealing Temperature | Amplicon Size, bp |
|---|---|---|---|
| 60ºC | 109 | ||
| Forward | 5׳-ACGAGATTGATGACCGCCTC | ||
| Reverse | 5׳-GCATCCACCCCACTCTCTTC | ||
| 60ºC | 143 | ||
| Forward | 5׳- GCCATCCCTGTCTTGCTCTT | ||
| Reverse | 5׳- TCCTCTCGGTCCAAGCCATA | ||
| 60ºC | 146 | ||
| Forward | 5׳-CGAGGTGATGCTTCTGGAGAC | ||
| Reverse | 5׳- TGGAGAACTCAAAGATGGGGTT | ||
| 60ºC | 143 | ||
| Forward | 5׳-ACTCGCAGGAAAGACTAGCA | ||
| Reverse | 5׳-AGCAGTGGAAGAATCGGACC | ||
| 60ºC | 199 | ||
| Forward | 5׳-GTGCCAGCCTCGTCTCATAG | ||
| Reverse | 5׳-GACTGTGCCGTTGAACTTGC |
4. Results
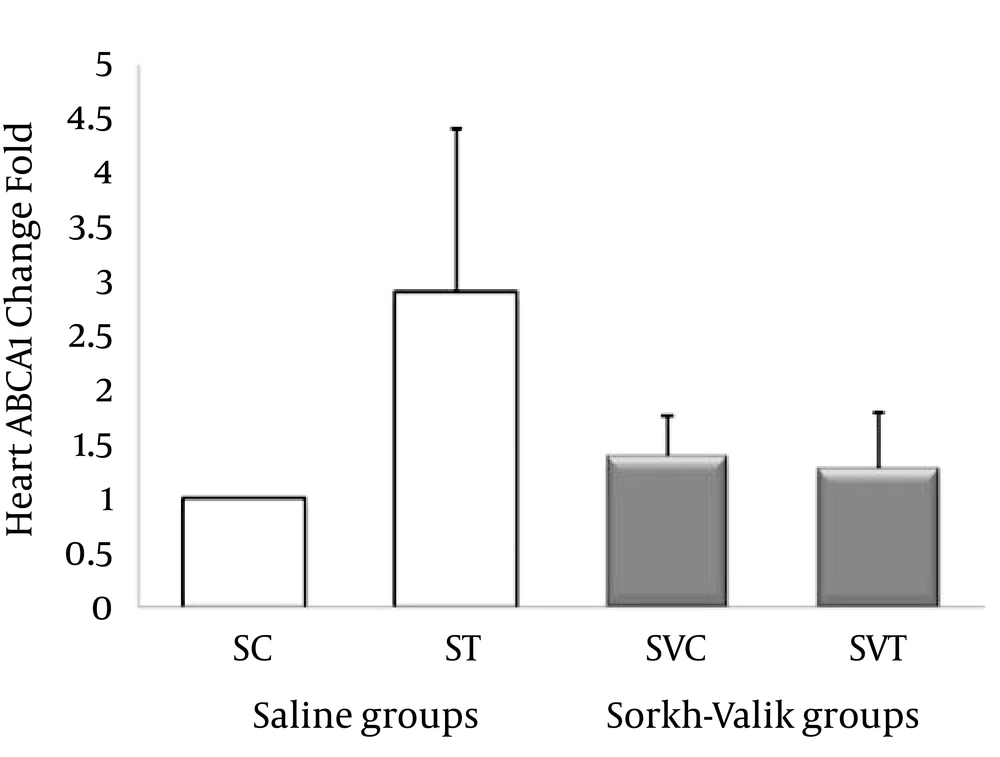
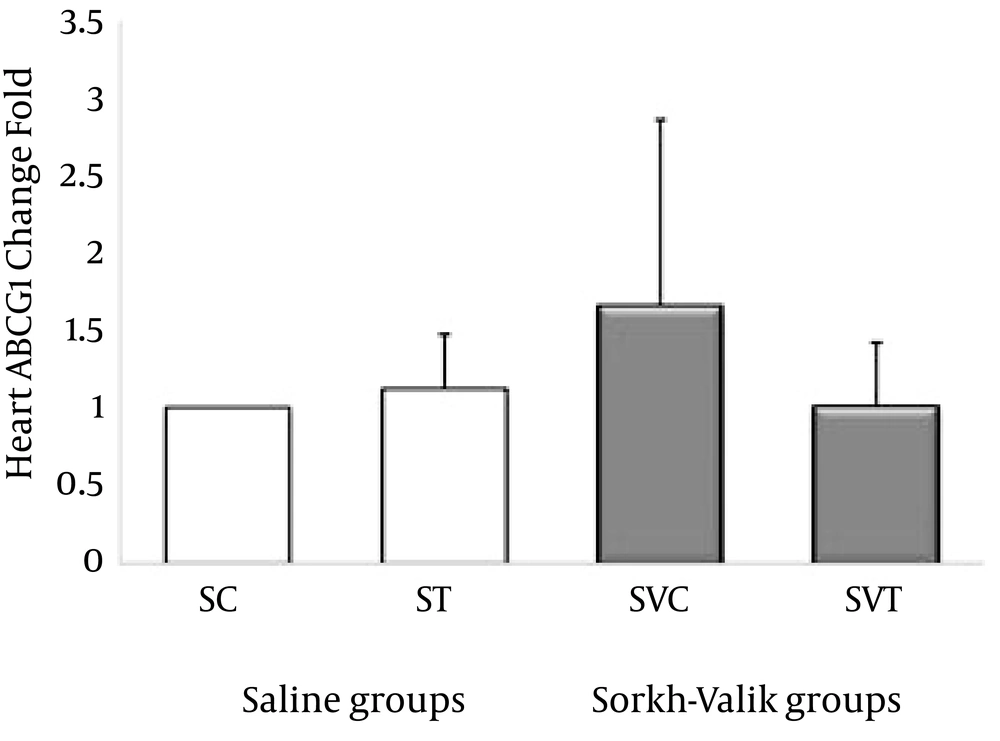
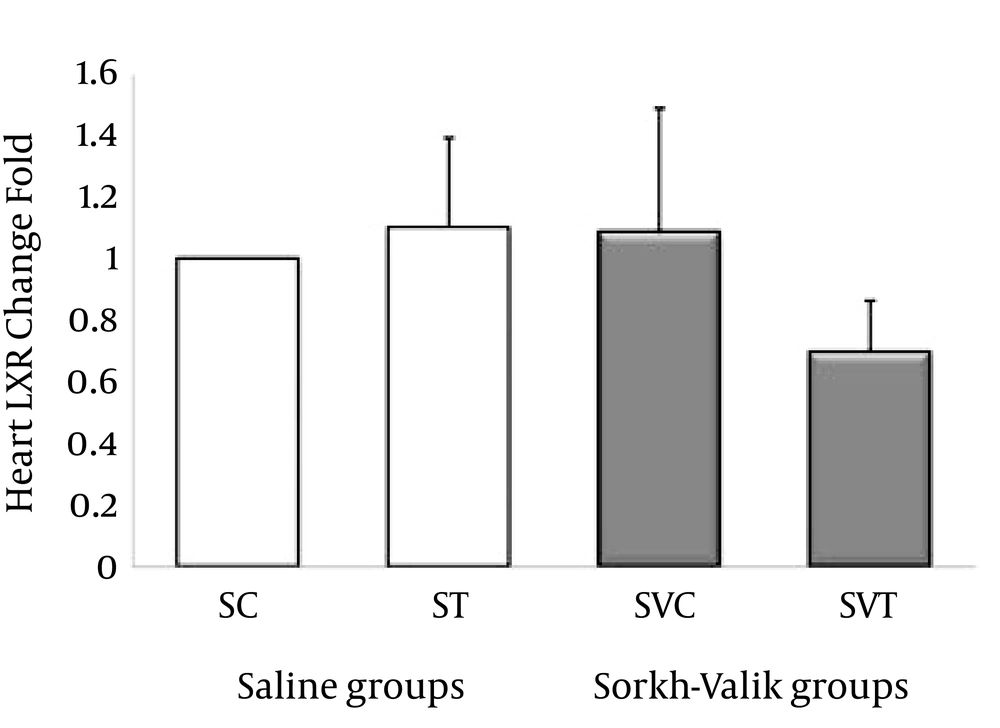
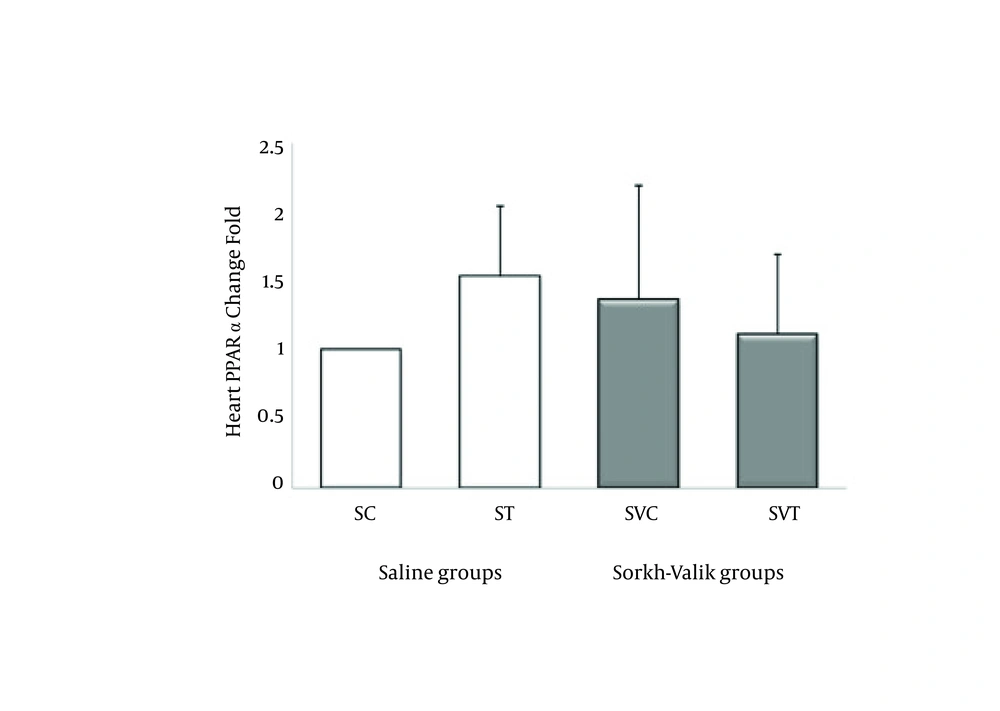
The ABCA1, ABCG1, LXR and PPAR-α genes were clearly expressed in rat heart tissue (Figure 1-4, respectively). The levels of ABCA1 and ABCG1 genes expression were not significant in response to training and supplementation (Table 2). However, the trend of changes show that the levels of ABCA1 not ABCG1 was higher in ST than SVT groups (Figures 1-2). Data also did reveal a lower and non-significant PPAR-α and LXR genes expression in trained Crataegus treated rats when compared with other groups, while the trend of changes indicates that LXR and PPAR-α mRNA expression were higher in saline-trained and Sorkh valik control groups. (Figures 3-4).
| Variables | Group | |||||
|---|---|---|---|---|---|---|
| SC | ST | SVC | SVT | F | P-Value | |
| 1 | 2.89 ± 1.51 | 1.39 ± 0.37 | 1.28 ± 0.50 | 1.08 | 0.3 | |
| 1 | 1.11 ± 0.36 | 1.65 ± 1.20 | 1.00 ± 0.41 | 0.22 | 0.8 | |
| 1 | 1.09 ± 0.29 | 1.08 ± 0.40 | 0.69 ± 0.16 | 0.50 | 0.6 | |
| 1 | 1.53 ± 0.51 | 1.36 ± 0.83 | 1.11 ± 0.58 | 0.17 | 0.9 | |
aData expressed as mean ± SEM.
5. Discussion
The results of current study indicate that ABCA1, ABCG1, LXR and PPAR-α mRNA were expressed in rat heart at rest and after training and Sorkh valik extraction supplementation. The results show that the trend of changes in the levels of ABCA1, LXR, and PPAR-α not ABCG1 mRNA expression was higher in saline-trained group. Other main finding was the reverse effect of exercise training on all determined gene expression in Sorkeh valik trained hearts. It should be noted that this is the first study to determine the effects of training program with Sorkh valik extract on ABCA1, ABCG1, LXR and PPAR-α mRNA expression in rat heart. Considering ABCA1, Ghanbari-Niaki et al. reported a higher and significant level of ABCA1 mRNA expression in rat liver after 12 weeks of endurance (25 m/min, 90 min/day) exercise training [3]. A higher and significant ABCA1 mRNA expression in rat heart and small intestine tissues were also reported by Khabazian et al. [17] and Ghanbari-Niaki [4] who employed the same treadmill running program but short duration (60 min/day). However, the lower ABCA1 mRNA after exercise training in Crataegus trained heart is not agreeing with our previous reports. In this study the level of ABCG1 remained unchanged in trained no control groups. We found a higher ABCG1 mRNA level in control-Crataegus treated hearts. Zare-Kookandeh et al. who observed a higher and significant levels of ABCG1 mRNA expression in rat small intestine and kidney tissues at the end of treadmill running program (25 m/min, 60 min/day, 5 days/week and for 8 weeks) in saline-trained not Baneh-trained groups [6]. A higher ABCG1 mRNA expression in saline-trained rat visceral fat by using the same treadmill running program was also reported by Ghanbari-Niaki et al. [18]. However, in these studies a lower ABCG1 mRNA expression was also observed in Pistacio atlantica (Baneh/Bene) treated an animal in this regards, our results are in consistent with above mentioned reports. With consider to the effect of Sorkh valik extraction on heart ABC families and selected nuclear receptors (LXR and PPARs) gene expressions, the information are very scarce. However, Xu et al. who reported hawthorn fruit compound reduced TG, LDL-C concentrations and TC/LDL-C ratio in mice treated with high fat diet [19]. In study by Zhang et al. supplementation with hawthorn fruit powder (Crataegus pinnatifida) (2 g/100 g of body weight) resulted in a reduction in plasma TC and TG no HDL levels in rabbit with high cholesterol diet. In addition, hawthorn powder also reduced TC concentration in rabbit heart and liver treated with high cholesterol diet [20]. Kwok et al. suggested that the lowering effect of hawthorn (Crataegus pinnatifida/shan zha) might be exert via up-regulation of hepatic CYP7A1 mRNA expression which leads to enhanced bile acid biosynthesis, and relieved HMG-CoA reductase suppression by high cholesterol diet [21]. In despite of these data, it is not clear that by which mechanism(s) SV extraction could increase rat heart ABCG1 not ABCA1 mRNA expression at rest not training. However, in the present study, a higher LXR and PPAR mRNA expression was observed in control-SV treated hearts. It seems that SV extraction might be exert an exercise-like effect on ABCG1 and LXR, PPAR in control-SV treated hearts. It is well known that PPARs act as nutritional lipid sensors that regulate a variety of homeostatic functions, including metabolism, inflammation and development. Three mammalian PPAR subtypes, designated PPAR-(NR1C1), PPAR-(NR1C3), and PPAR-(NR1C2) have been identified [22]. It has also suggested that PPAR activity in cardiac muscle is an important regulator of mitochondrial fatty acid uptake and oxidation with significant myocardial lipid accumulation observed in PPAR knock-out mice [22]. It should be noted the adult heart normally obtains 50-70% of its ATP from fatty acid β-oxidation and on a physiological condition, FA is considered to account for 60-70% of oxygen consumption for energy production in the heart and high intensity exercise might reduce FA metabolic capacity and increase nonfat oxidation capacity such those happened in aged rats [23, 24]. Thus a lower PPAR and LXR mRNA expression in SV-trained heart might be due to a reduction in FA metabolism capacity and the fuel switching from fatty acid oxidation to carbohydrate metabolism [25, 26], particularly lactate consumption as a fuel for energy provision in high-intensity and long-term exercise training [27].
In summary, the present results indicate that ABCG1, LXR and PPAR not ABCA1 mRNA expression are responding differently to Sorkh valik extraction at rest and following a high intensity (34 m/min) treadmill running program. Data also indicate that under our experimental condition SV extraction was able to enhance the levels of ABCG1, LXR and PPAR mRNA expression at rest not training. Further studies are warrant to clarify the effects of different intensities low (10 m/min) to very high intensities (50 m/min) on above mentioned gene expression.