1. Background
Pseudomonas aeruginosa is one of the resistant bacteria to antibiotics. This Gram-negative and opportunist bacterium causes a broad spectrum of diseases, including bacteremia and infection of the respiratory system, bones, and joints (1). Numerous studies have reported that the main problem in clinical treatments is the evolution of antibiotic resistance among P. aeruginosa strains (2). Therefore, new therapies are required to replace conventional antibiotic therapies.
By introducing P. aeruginosa into the body, the immune system is stimulated. Moreover, P. aeruginosa, by releasing reactive oxygen species (ROS), invites immune cells and protects the body against foreign agents. By increasing ROS in the body, the balance between the antioxidant and body oxidative states is disrupted leading to weakening the immune system (3). Therefore, by increasing the level of antioxidants in the body, it is possible to strengthen the immune system against pathogens.
Several studies have analyzed the positive effects of numerous herbal ingredients. Kavyani et al. demonstrated the inhibition of the LasI/LasR quorum sensing system and the prevention of ROS increase using cinnamon extract against P. aeruginosa infection (4). In addition to plants, numerous probiotics can be good candidates for such studies. Probiotics are living microorganisms that are part of the natural gut flora. Consuming a certain amount of probiotics can be beneficial for the body. One of the most important properties of probiotics is strengthening the immune system due to their high antioxidant properties. Probiotics include lactobacilli and yeasts (5).
The antioxidant and antibacterial effects of Saccharomyces cerevisiae as a probiotic have been confirmed in studies performed by Khanian et al., Vazquez et al., and Fakraddin et al. The antibacterial capability of S. cerevisiae might be due to the secretion of inhibitory proteins and extracellular protease, stimulation of immunoglobulin A, and elimination of sulfur dioxide (6-8). Several studies have also attributed the antioxidant properties of yeasts to their wall polysaccharides, β-D-glucans, and α-D-mannans (9).
Selenium is a rare and essential element in the body with strong antioxidant properties, similar to probiotics. Selenium is crucial as a cofactor for glutathione peroxidase (GPx) and the principal component of selenoenzymes. The GPx, with high antioxidant properties, plays a key role in all living tissues and can prevent harmful oxidation in the cell by reducing cell peroxides. At the center of all these enzymes, there is the amino acid selenocysteine, which acts as an oxidizing and reducing agent. Therefore, selenocysteine plays a role in protecting against cellular toxicity. The function of this element in the antioxidant defense system causes the body to pass through the conditions of oxidative stress (10). Therefore, this study investigated the antioxidant effects of selenium-enriched S. cerevisiae against oxidative stress induced by P. aeruginosa.
2. Objectives
This study aimed to investigate the antioxidant levels and hematological parameters of rats infected with P. aeruginosa after treatment with S. cerevisiae and selenium-enriched S. cerevisiae.
3. Methods
3.1. Animal Grouping
A total of 36 female mice (weight range: 100-150 g) were fed under a 12 h light/12 h dark cycle, 51 ± 5% humidity, and 23 ± 3°C temperature. For further investigation, these mice were classified under six groups. The control group (group A) was fed only with water and food for 4 weeks with the intragastric administration of 0.5 mL physiologic serum by gavage every other day. Saccharomyces cerevisiae-treated rats (group B) received the intragastric administration of 0.5 ml yeast suspension for 4 weeks by gavage every other day (0.5 × 107 CFU/mL). Selenium-enriched S. cerevisiae-treated rats (group C) received the intragastric administration of selenium-enriched yeast suspension by gavage every other day (0.5 × 107 CFU/mL) for 4 weeks. The infected rats with P. aeruginosa were treated with S. cerevisiae (group D) intragastrically by gavage every other day and received 0.5 ml intraperitoneal P. aeruginosa at the end of the third week (1.5 × 108 CFU/mL) (ATCC 27853). Similar to group C, the infected rats with P. aeruginosa were treated with selenium-enriched S. cerevisiae (group E) intragastrically by gavage every other day and received 0.5 mL intraperitoneal P. aeruginosa (1.5 × 108 CFU/mL) at the end of the third week. The rats in group F were fed with water and food for 4 weeks and received 0.5 mL intraperitoneal P. aeruginosa at the end of the third week (1.5 × 108 CFU/mL).
3.2. Saccharomyces cerevisiae Enrichment with Selenium
Saccharomyces cerevisiae (ID: PTCC 5177) were purchased from the collection center of industrial microorganisms of Iran, and the standard suspension was provided. For enrichment, 90 μl sodium selenite was added to 100 μL standard yeast suspension (0.005 mg/mL) and incubated for 48 h at 37°C. Subsequently, the suspension was washed by normal sterile saline and centrifuged at 3000 rpm for 15 min. For the removal of extra selenium, the pellet was washed three times with normal sterile saline serum and diluted to 0.5 × 107 CFU/mL (11).
3.3. Evaluation of Antioxidant Factors
After the treatment period, the animals were anesthetized to take blood samples directly from their hearts. The Mindray BC-6800 cell counter was used to count the blood cells and measure their indices. The total antioxidant capacity (T-AOC) was measured by Eastbiopharm Kit (Eastbiopharm Co., Ltd., China) according to the manufacturer’s protocol. The T-AOC test kit is based on an antibody sandwich technique and provides a simple, repeatable, and standardized method for the measurement of the T-AOC in biological samples, such as serum, blood plasma, saliva, and other body fluids. Glutathione (GSH) concentration was measured by ZellBio GmbH Kit (ZellBio GmbH, Germany). The GSH kit is used to determine GSH activity within the range of 0.03-1 mM. This kit works by GSH reaction with 5,5′-dithiobis-(2-nitrobenzoic acid), which results in 2-nitro-5-benzoic acid production producing a yellow substrate. This kit also contains 5-sulfosalicylic acid to remove proteins from the samples and protect the GSH oxidation and γ GSH transpeptidase reaction. Additionally, GPx activity was measured by Zellbio GmbH Kit according to the manufacturer’s protocol. The GPX activity assay kit can determine GPX in biological samples with 5 mL sensitivity (5 KU/L). In this assay, the GPX activity unit was considered the amount of the sample that will catalyze the decomposition of 1 µmole of GSH to glutathione disulfide in 1 min.
3.4. Statistical Analysis
The data were presented as mean ± standard deviation. After ensuring the normality of the data according to the Kolmogorov-Smirnov test, all the data were analyzed by SPSS software (version 21, IBM, USA). A one-way analysis of variance was used to examine the significant differences in means, and then the Tukey posthoc test was used to investigate intergroup comparisons. The significant level was considered less than 0.05.
4. Results
According to Table 1, the number of white blood cells (WBC) in the P. aeruginosa infected group was significantly higher than that of the control group (P = 0.01). The number of WBC significantly decreased in the S. cerevisiae and selenium-enriched S. cerevisiae group and selenium-enriched S. cerevisiae infected group, compared to that of the infected group (P = 0.005, P = 0.001, and P = 0.006, respectively).
| Parameter | Experimental Groups | |||||
|---|---|---|---|---|---|---|
| Control | Saccharomyces cerevisiae | Saccharomyces cerevisiae + Selenium | Pseudomonas aeruginosa + Saccharomyces cerevisiae | Pseudomonas aeruginosa + Saccharomyces cerevisiae + Selenium | Pseudomonas aeruginosa | |
| WBC (103/µL) | ##6.60 ± 1.499 | ##6.41 ± 1.404 | ###5.91 ± 1.404 | 8.883 ± 0.913 | ##6.466 ± 1.489 | 9.45 ± 1.234** |
| RBC (106/µL) | 7.86 ± 0.454 | 7.98 ± 0.859 | 7.73 ± 0.335 | 7.98 ± 0.856 | 7.63 ± 0.579 | 6.87 ± 1.226 |
| Hgb (gr/dL) | 14.23 ± 0.697 | 14.88 ± 1.241 | 14.62 ± 0.435 | 14.32 ± 0.690 | 15.38 ± 1.683 | 13.30 ± 2.089 |
| Hct (%) | 44.70 ± 2.546 | 46.95 ± 5.975 | 45.23 ± 1.406 | 48.57 ± 8.695 | 48.18 ± 6.407 | 39.05 ± 4.412 |
| MCV (f L) | 58.85 ± 1.096 | 57.75 ± 1.724 | 58.52 ± 1.03 | 58.57 ± 1.920 | 59.57 ± 3.661 | 61.53 ± 7.020 |
| MCH (pg) | 18.12 ± 0.549 | 18.40 ± 1.218 | 18.90 ± 0.384 | 18.48 ± 0.649 | 19.12 ± 1.832 | 19.42 ± 1.552 |
| MCHC (g/dL) | 31.86 ± 0.427 | 31.83 ± 1.389 | 32.32 ± 0.365 | 31.57 ± 0.598 | 32.03 ± 1.172 | 31.67 ± 1.583 |
| PLT (105/µL) | 8.72 ± 0.991 | 8.97 ± 0.944 | 8.70 ± 0.917 | 8.04 ± 1.521 | 7.10 ± 1.963 | 9.39 ± 1.38 |
Abbreviations: WBC, white blood cells; RBC, red blood cells; Hgb, hemoglobin concentration; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count.
a Values are expressed as mean ± standard deviation.
bLevels of significance values are *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001. The asterisks indicate the significance levels of experimental groups in comparison to those of the control group, and # indicate the comparison of experimental groups compared to those of the infected group.
There was no significant difference in the number of red blood cells (RBC), platelet count (PLT), hemoglobin concentration (Hgb), hematocrit (Hct), mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration indices among the experimental groups, compared to those reported for the group control.
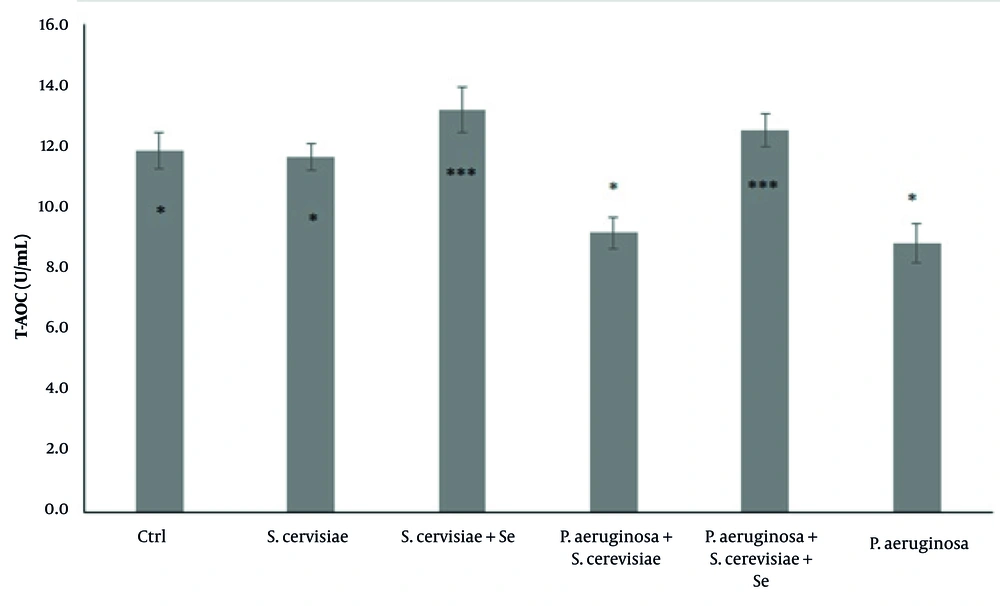
Although the T-AOC was significantly increased in the selenium-enriched S. cerevisiae (P = 0.001) and the selenium-enriched S. cerevisiae infected (P = 0.001) groups, compared to that of the infected group (P = 0.014), it was significantly decreased in the P. aeruginosa infected and S. cerevisiae infected (P = 0.957) groups (Figure 1), compared to that of the control group.
Comparison of total antioxidant capacity among experimental groups. The values are expressed as mean ± standard deviation. The above asterisks within the figure are the results of statistical analysis of the comparison between the experimental groups and the control group. The beneath asterisks are the statistical analysis results of the comparison between the experimental groups and the P. aeruginosa-infected group. Ctrl, Control; S. cerevisiae, Saccharomyces cerevisiae; Se, Selenium; P. aeruginosa, Pseudomonas aeruginosa.
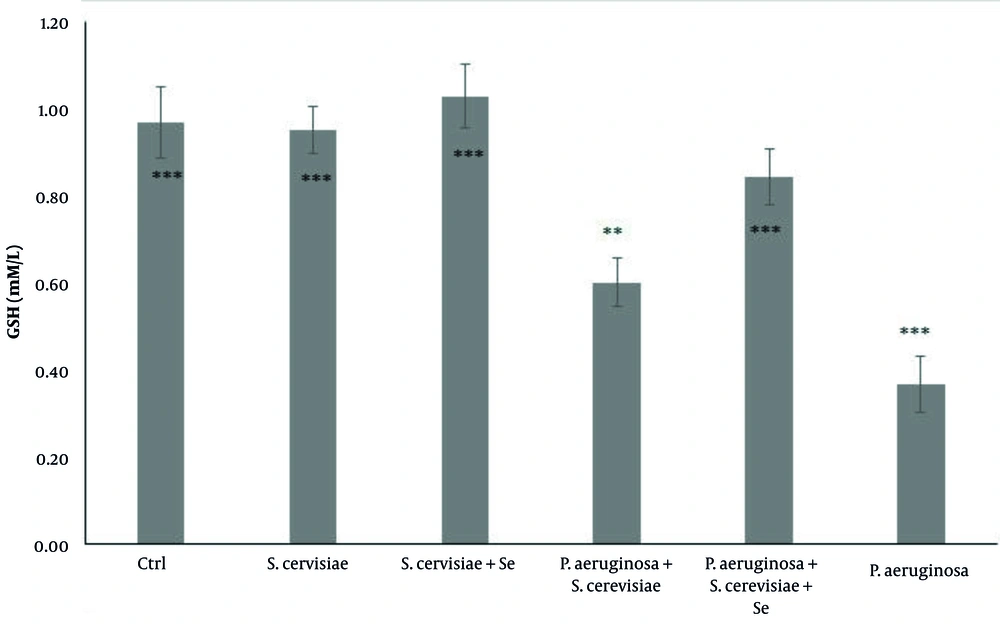
Although an increase was observed in GSH in the selenium-enriched S. cerevisiae infected (P = 0.001), selenium-enriched S. cerevisiae treated (P = 0.01), and S. cerevisiae treated groups (P = 1), compared to that of the infected group, its concentration was significantly decreased in the selenium-enriched S. cerevisiae infected (P = 0.765), and P. aeruginosa infected (P = 0.001) groups (Figure 2), compared to that of the control group.
Comparison of glutathione concentration among experimental groups. The values are expressed as mean ± standard deviation. The above asterisks within the figure are the results of the comparison of the experimental groups with the control group. The beneath asterisks are the results of the comparison of the experimental groups with the P. aeruginosa-infected group. Ctrl, Control; S. cerevisiae, Saccharomyces cerevisiae; Se, Selenium; P. aeruginosa, Pseudomonas aeruginosa
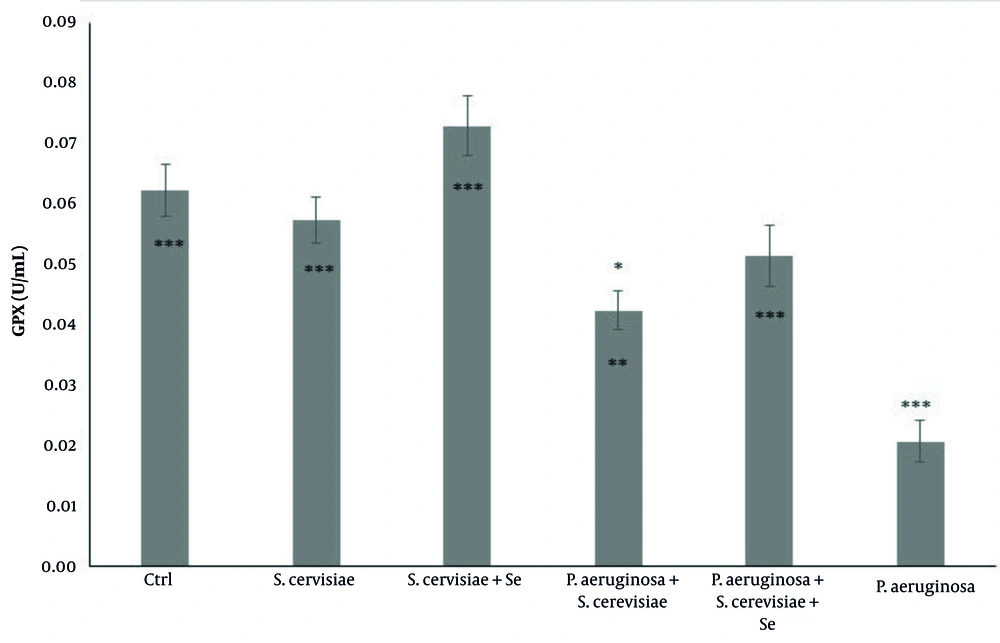
The GPX activity in the S. cerevisiae infected group (P = 0.011), selenium-enriched S. cerevisiae infected group (P = 0.001), selenium-enriched S. cerevisiae-treated group (P = 0.001), and S. cerevisiae-treated group (P = 0.001) showed a significant increase, compared to that reported for the infected group. The activity of this enzyme in the infected group (P = 0.001) was significantly decreased compared to that reported for the control group (Figure 3).
Comparison of glutathione peroxidase activity among experimental groups. The values are expressed as mean ± standard deviation. The above asterisks are the results of the comparison of the experimental groups with the control group. The beneath asterisks are the results of the comparison of the experimental groups with the P. aeruginosa-infected group. Ctrl, Control; S. cerevisiae, Saccharomyces cerevisiae; Se, Selenium; P. aeruginosa, Pseudomonas aeruginosa
5. Discussion
In the present study, the T-AOC in the P. aeruginosa infected rats significantly decreased compared to that of the control group (Figure 1). These changes can indicate an increase in the level of oxidative stress in the body. In line with the aforementioned results, van‘t Wout et al. reported the increased expression of the genes associated with oxidative stress, such as C/EBP homologous protein (CHOP), 78-kDa glucose-regulated protein (GRP78), and Growth arrest and DNA damage-inducible protein 34 (GADD34), after the infection of lung epithelial cells with P. aeruginosa (12). In another study, Liu et al. observed an increase in ROS levels after infecting the A549 cell lines (alveolar epithelium) with the lipopolysaccharides (LPS) wall of P. aeruginosa (13).
The entrance of pathogens into the body induces oxidative stress, which weakens the antioxidant system and decreases the antioxidant level (10). The present study confirmed the decrease of the antioxidant level after introducing the P. aeruginosa and oxidative stress response. Consistently, reducing the antioxidant capacity following the Pseudomonas infection confirms the oxidative stress in the body.
There was a significant increase in the T-AOC level following the treatment of P. aeruginosa-infected rats with selenium-enriched S. cerevisiae, compared to that of the infected group (Figure 1). In this study, it appears that S. cerevisiae enriched with selenium prevents the induction of oxidative stress following the entry of pathogens into the body. A recent study showed the protective role of selenium-enriched probiotics in the inhibition of Escherichia coli pathogenesis and the improvement of antioxidant status. (14).
Zhu et al. investigated the effect of yeast S. cerevisiae supplementation on serum antioxidant capacity and gut microbial populations in weaned piglets. The results of the aforementioned study showed an increase in serum superoxide dismutase (SOD) activity and a decrease in serum malondialdehyde concentration (P < 0.05). Zhu et al. suggested that various forms of yeast with very important antioxidant enzyme systems, such as SOD or catalase, may have moderated the body’s antioxidant capacity and boosted the intestinal immunity of weaned piglets (15).
Kleniewska et al. studied the effects of Lactobacillus casei and inulin prebiotics on the antioxidant capacity of human plasma. They showed a significant increase in catalase and a slight increase in the activity of SOD and GPx (16). Roshan et al. examined the effect of probiotics and synbiotics on antioxidant status. They demonstrated that these supplements improve GSH as critical markers of antioxidant status in the body (17). Nido et al. reported that the body weight and liver malondialdehyde level of mice fed a high-fat diet decreased significantly after treatment with selenium enriched S. cerevisiae (18). A recent study demonstrated the antibacterial and antioxidant features of selenium-enriched Enterococcus durans LAB18s against P. aeruginosa pathogenesis (19).
Various studies have shown that selenium-containing compounds increase the expression of selenoprotein-associated genes and decrease the level of oxidative stress biomarkers in the body (20). Alyemeni et al. demonstrated that after selenium treatment, less amount of cadmium would be absorbed in tomatoes, and selenium treatment reduced the cadmium-induced oxidative stress in tomatoes by increasing the activity of antioxidant enzymes (21). Yaghchi et al. reported that the probiotic selenium-enriched Saccharomyces boulardii attenuates oxidative stress in rats after aluminum toxicity (10).
It has also been proven that DL-selenomethionine can have protective effects on hepatocytes against oxidative stress induced by T-2/HT-2 toxins-induced cytotoxicity. This protective role is accomplished by increasing the expression of antioxidant genes and activity of GPX and GSH and decreasing the intracellular ROS level (22). In addition, selenium, as a biocatalytic and functional component of the GPx family and selenoproteins, plays an important role in the detoxification of a wide range of peroxides, such as hydrogen peroxide (23).
The results of the above-mentioned studies could indicate why the level of serum antioxidants in rats treated with selenium-enriched yeast significantly increased, compared to that reported for selenium-free yeast. Increased GSH levels and GPx activity (Figures 2 and 3) also confirmed the positive effect of selenium and yeast on the inhibition of oxidative stress induced by P. aeruginosa. In the confirmation of these results, Binte Hussain et al. demonstrated increased GSH level and GPX activity after sodium selenite treatment following oxidative stress induction by cadmium in PC12 cells (24). Another study reported the protective effect of selenium-enriched yeast against oxidative stress and liver inflammation caused by poisoning with carbon tetrachloride in rats (25).
In the present study, there was no significant difference in the T-AOC, GSH concentration, and GPX activity between the yeast and selenium-enriched yeast treated groups, compared to those of the control group, indicating that yeasts have no adverse effect on the rats’ bodies (Figure 1, 2, and 3). Blood parameters were evaluated and compared between the experimental groups to ensure that the dose of yeast and selenium was safe for the body. The results showed that none of the blood parameters was influenced by selenium-enriched S. cerevisiae and S. cerevisiae (Table 1). According to the results, the collected data have reported a lack of changes in the number of PLT, Hgb, Hct, RBC, and indices following the use of probiotics, such as Kocuria SMI, Rhodococcus and selenium-enriched S. cerevisiae (26), and Lactobacillus reuteri KT260178 and selenium-enriched S. cerevisiae (27). Mandour et al. showed that the supra-nutritional dose of selenium-enriched yeast had no clinical or laboratory toxicity in male goats (23).
The evidence showed that selenium supplement in Tilapia increased Hgb level, weight, and body length; however, it did not significantly alter the number of RBC and WBC. The aforementioned data suggested that selenium did not change blood factors in Tilapia; nevertheless, selenium improves physiological function and fish growth (28). Wang et al. demonstrated that the probiotic Lactobacillus johnsonii BS15 inhibits subclinical necrotic enteritis in chickens by improving blood parameters related to the immune system. Furthermore, the BS15 supplement improves blood parameters in healthy chickens, especially at the starter phase (29). Variations in the impact of selenium-enriched probiotics on blood parameters might be attributed to the differences in dietary composition, probiotic dose, mode of administration, and animal species (30).
In the current study, the number of WBC significantly increased only in the group infected with P. aeruginosa, compared to that reported for the control group, which could be due to the stimulation of the immune system following the penetration of the pathogen into the body. The treatment of the infected rats by yeast and selenium-enriched yeast significantly caused a reduction in WBC count near the control level. The aforementioned results confirm the protective effect of this probiotic against P. aeruginosa. It seems that through strong antioxidant effects, probiotics enhance the immune system to fight against pathogens (Table 1).
5.1. Conclusions
The results of the present study showed that the entry of P. aeruginosa stimulated immune cells and increased ROS to fight pathogens resulting in the induction of oxidative stress and a decrease in the level of natural antioxidants in the body. Selenium and S. cerevisiae, due to their strong antioxidant properties, somewhat reduce the harmful effects of oxidative stress on the bodies of pathogen-infected groups. No change in the blood factors in the experimental groups confirmed the safety of selenium-enriched yeast for the bodies of the treated groups. Therefore, further studies are required to confirm the aforementioned results.