1. Background
The neurolinguistics, which deals with the relationship between the brain and language, provides a sound basis for studying how the brain works when understanding, communicating, and producing language (1).
Franka divides neurolinguistics into two areas of language disorder and the learning process. Franka believes that linguistic deficits dates back to 400 BC (2). Another area of neurolinguistics dealing with the language in a healthy brain emerged in the 1950s when Chomsky gave his generative grammar and introduced the origin of language. It is assumed that language disorders result from the damage to specific areas of the brain hemisphere. These injuries can cause problems to speech, understanding speech, and writing. The language loss as a result of local injury to the brain has attracted the attention of researchers for more than one and half a century and led to the emergence of a new science called aphasiology. The study of language disorders caused by brain injury has, in turn, led to the emergence of theories concerning the basic cognitive processes of language and the way that language is represented and processed in the brain (3). Parkinson’s disease (PD) is a group of extracorporeal neurodegenerative diseases in which the midbrain cells are gradually destroyed and their dopamine production decreases. It is accompanied by manifestations of body tremors (at rest), walking disorders, slowness of motion, stiffness, and dryness. In 80% of the cases, PD can present in addition to the aforementioned physical symptoms with cognitive impairment and dementia, and some believe Parkinson’s disease with dementia (PDD) can be considered as independent of PD. Lewy body dementia characterized by progressive dementia of cognitive fluctuations, delirium and, finally, some Parkinson’s features differs from dementia following Parkinson’s in late onset of moving disorders in Lewy body dementia (4-6).
Given the similarities between these three diseases and based on the only studies conducted by Ash et al., these three diseases can be considered as the range of Lewy body spectrum disorders (LBSD) (7, 8).
Various studies have explored the types of speech and speech disorders in Parkinson’s patients with and without dementia (9-12); however, only a few studies have investigated the linguistic disorders of Lewy body dementia based on data about patients with LBD such as Parkinson’s patients with dementia who had linguistic abnormalities at higher levels than Parkinson’s patients without dementia due to dementia-related effects. In these patients, a range of speech disorders has been reported including speech production problems, speech disorders, lack of executive skills, as well as difficulty in retrieving correct vocabulary and grammar (7, 8).
According to some studies, disorders in each of these domains (e.g., executive skills) have been linked to various causes, including anatomical light variations. Furthermore, extensive studies have investigated the various aspects of linguistic disorders of Lewy body dementia and its possible causes, as well as its similarities and differences with dementia following Parkinson’s.
2. Objectives
Given the above discussion, the present study aimed to investigate speech disorders in groups of patients with Lewy-body spectrum disorders, compare the groups in order to identify their possible causes, and improve the quality of life and rehabilitation of these patients by managing the development of speech disorders.
3. Methods
This case-control study investigated a total of 40 individuals, including 10 healthy controls and 30 patients with a range of LBSD diagnosed based on the criteria for cognitive neurological and motor disorders published by the University of Pennsylvania Department of Neurology confirmed by a neurologist; at least two years had elapsed since the onset of their disease (13).
Initially, patients were divided into three groups of 10 patients with LBSD, including PD patients, patients with PDD, and patients with dementia with Lewy bodies (DLB), who were matched as closely as possible. The diagnosis of PDD was established for patients when the movement symptoms appeared at least one year before the onset of dementia (14). However, the diagnosis of LBD at the onset of dementia with one-year precedence was determined to be associated with movement symptoms. Other diagnostic features of LBD were considered based on the criteria of the third LBD consortium, which included cognitive fluctuations, changes in consciousness and concentration, as well as visual hallucinations (albeit mildly interfering with tests) (15). Patients were evaluated based on, in addition to clinical criteria, short mental status test (MMSE equal to or less than 24) (16). Demographic information and clinical characteristics of patients, including age, sex, MMSE score, medication history in Czech, a list of information about each patient, and the results of linguistic tests were collected. Exclusion criteria were dementia caused by other factors such as metabolic, endocrine, vascular, structural, nutritional, infectious, and primary mental disorders. Each group of patients was then evaluated for linguistic status using a 24-page story narrative. Each patient was asked to glance at the book and become familiar with its content and then narrate the story of the book as it is narrated for children. The evaluator did not interrupt the patient during the presentation of the narrative, and the whole narrated story was first transcribed and then transcribed carefully by a trained linguist and a linguist specialized in grammar and discourse analysis. These analyses included storytelling duration (in seconds), number of sentences, number of vocabulary retrieval problems, number of keywords, frequency of incomplete content, frequency of action narrative, frequency of global and local connectedness, and frequency of incomplete search theme in each group. It was isolated and compared with other groups. Finally, the results of these analyses, as well as the patients’ baseline information and their mental and neurological status were entered into SPSS software version 22. Then the groups were compared using statistical tests by the respected statistician.
4. Results
This case-control study was carried out to evaluate language disorders in patients with a range of LBSD and healthy controls. The four groups were similar in age and sex distribution, and had no significant difference. The highest score of the short mental state test (MMSE) was obtained for the control group, followed by Parkinson’s and Parkinson’s patients with dementia. Patients with Parkinson’s Lewy body obtained the lowest score in short mental status test, and this score was significantly different for the four groups according to the Kruskal-Wallis test (P < 0.0001).
The time spent for storytelling in the control group was, on average 115.9 ± 219.4 seconds. The shortest storytelling time was obtained by Parkinson’s patients with duration of 111.9 ± 430.4 seconds, followed by Lewy body patients with 110.7 ± 477.6 seconds duration. Parkinson’s patients with dementia also spent the longest time to tell the story. There were also statistically significant differences among study groups regarding the time spent for storytelling according to the Kruskal-Wallis test (P < 0.0001).
The average numbers of sentences in the control group and dementia with Lewy body were lower than those in the other two groups, and the highest number was 9.5 ± 58 obtained by the group of patients with PD. There were also statistically significant differences among study groups regarding the number of sentences according to post-hoc Dunn test (P = 0.007). The results showed that there was a significant difference between the control group with dementia and Parkinson concerning the number of sentences (P = 0.05, P = 0.009, respectively). However, no statistically significant difference was detected between other groups.
As for the narrative, the control group (healthy) had the least number of vocabulary retrieval problems, averaging 1.3 ± 1.4 per a total of 14.8 ± 40 words. On the other hand, Parkinson’s patients with dementia had the most difficulty in vocabulary retrieval with 17.9 ± 57 words, averaging 10.5 ± 19.2 vocabulary retrieval problems. Vocabulary retrieval was most prevalent in Parkinson’s patients with 17.2 ± 13.3 words and then in Lewy body Parkinson’s patients with a mean of 14.6 ± 8.3 words. According to the Kruskal-Wallis test results, there were also statistically significant differences among the study groups regarding the number of vocabulary retrieval problems (P < 0.0001).
Frequencies of incomplete content were 1, 7, 2, and 4 cases in healthy subjects, Parkinson’s patients, Parkinson’s patients with dementia, and dementia with Lewy body, respectively. Furthermore, 9 subjects in the control group, 3 in the Parkinson group, and 2 in the dementia group provided full story content; while 4 patients in the dementia group and 4 patients in the Lewy body misunderstood the content and two individuals from the two groups failed to understand the story. In general, according to Fisher’s exact test results, there were also significant differences among the four groups in terms of the content mentioned (P < 0.001).
The frequency of action narrative in the story was nine cases in healthy subjects, three cases in Parkinson’s patients, and two cases in Parkinson’s patients with dementia. In addition, one patient in the control group, seven in the Parkinson group, eight in the dementia group, and four patients with dementia with Lewy body in the story were incomplete. However, six out of 10 patients in the Lewy body group did not observe the action in the story. In general, according to Fisher’s exact test results, there were also significant differences among the four groups regarding the mentioned practice (P < 0.001).
Frequencies of incomplete search were 0, 6, 8, and 4 in healthy subjects, Parkinson’s patients, Parkinson’s patients with dementia, and dementia with Lewy body, respectively. All control subjects, 4 Parkinson’s and 2 dementia subjects retained the search theme. However, 6 patients in the Lewy body group did not search the theme. In general, according to Fisher’s exact test results, there were also significant differences among the four groups in terms of subject searching (P < 0.001).
All control subjects, 7 patients with PD, 4 patients with Parkinson’s with dementia, and 2 patients with Lewy body had global connectedness in storytelling, whereas 3, 6, and 8 patients from Parkinson, dementia, and Lewy body groups, respectively, did not have a global connectedness. In general, according to Fisher’s exact test results, there were significant differences among four groups in terms of the global connectedness of storytelling (P = 0.001).
Nine subjects in the control group, 3 in the Parkinson group, and 2 in the Parkinson with dementia group were able to establish local connectedness in the narrative with 1, 7, 8, and 4 patients in the control, Parkinson, dementia, and Lewy body groups, respectively. The narrative had a local connectedness story. Six of the patients in the Lewy body group failed to establish local connectedness completely. In general, according to Fisher’s exact test results, there were significant differences among four groups in term of the local connectedness of storytelling (P < 0.001).
As for the number of keywords used by people in storytelling, the most and the least mentioned keywords in the control group was 3.9 ± 27.9, and in the Parkinson’s patients Lewy body was 6.5 ± 6.6. The number of words used by patients with Parkinson’s and dementia were approximately 9.4 ± 18.8 and 9.4 ± 18, respectively. There were also statistically significant differences among the study groups regarding the number of keywords used according to the Kruskal-Wallis probability test results (P < 0.001).
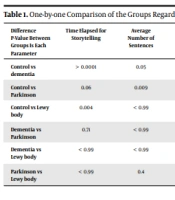
Since the differences among the groups were significant, they were compared with one another in all respects. Interestingly, there was no difference between dementia and Parkinson groups regarding the various parameters of our study. Table 1 shows the results of this one-by-one comparison.
| Difference P-Value Between Groups Is Each Parameter | Time Elapsed for Storytelling | Average Number of Sentences | Number of Vocabulary Retrieval | Incomplete Content | Action Narrative | Frequency of Incomplete Search | Global Connectedness in Storytelling | Local Connectedness in the Narrative | Keywords Used |
|---|---|---|---|---|---|---|---|---|---|
| Control vs dementia | > 0.0001 | 0.05 | > 0.0001 | 0.005 | 0.005 | 0.001 | 0.011 | 0.005 | 0.123 |
| Control vs Parkinson | 0.06 | 0.009 | 0.002 | 0.02 | 0.02 | 0.011 | 0.211 | 0.02 | 0.09 |
| Control vs Lewy body | 0.004 | < 0.99 | > 0.0001 | > 0.0001 | > 0.0001 | > 0.0001 | 0.001 | 0.0001 | > 0.0001 |
| Dementia vs Parkinson | 0.71 | < 0.99 | < 0.99 | 0.019 | < 0.99 | 0.628 | 0.37 | < 0.99 | < 0.99 |
| Dementia vs Lewy body | < 0.99 | < 0.99 | < 0.99 | 0.539 | 0.011 | 0.011 | 0.628 | 0.011 | 0.162 |
| Parkinson vs Lewy body | < 0.99 | 0.4 | < 0.99 | 0.011 | 0.009 | 0.003 | 0.07 | 0.009 | 0.211 |
5. Discussions
According to the results of this study, significant speech disorders were observed in patients of all three groups. Patients with dementia with Lewy body had the lowest production rate and produced narratives with the least words per minute. Patients with PD and Parkinson with dementia also had difficulty finding the words they needed to tell the story. Although patients with PD were less likely to find vocabulary, they could not effectively express their views on the story organization, which may have been due to their poor performance in measuring executive resources requiring organized mental search. These results were in line with those obtained in the only published study by Ash et al. (8) in which patients with dementia had the most language impairment compared to other groups. Ash et al. also used imaging techniques for investigating the structural causes and confirming the results, and found that the correlation between story organization and frontal cortical atrophy was stronger in the right and posterior brain regions of the patients with PD (7, 8).
Slowness is a prominent feature of patients with Parkinson with dementia and dementia with Lewy body. Patients with PD have difficulty in expressing story images as well as in remembering and naming words when the speaker is looking for a particular word. In this case, the announcer is finally able to achieve his desired form after a delay, while Parkinson’s patients with dementia make no effort to find the exact word and sometimes use a general noun instead of specific one.
Our study results demonstrated that the patients in control group were more accurate in expressing the accidents and reported less frequent events than the Parkinson’s and dementia patients. Patients with dementia had the most errors in this regard.
One of the variables examined in the present study was the content errors in storytelling by different groups. The results showed that patients with dementia had the least effort and success in terms of internal consistency. Those in control group sometimes omitted the events and made content errors when describing the events, but they made more obvious errors in relating the events not described before.
In the present study, the control group was very successful in performing a complex set of tasks required to produce a coherent narrative. All three groups of patients showed deficits in performing these tasks compared to each other. In addition, the nature of their disorders was relatively different in each subgroup of patients. Patients with PDD appeared to have more difficulty in retrieving the words they needed to present a narrative. Although they used words alone to describe an image or found the required rules to combine words and sentences, their narratives lacked the elements of global and local connectedness to unite the elements of the story into one whole story. Coherence is essential in storytelling. Patients with simple Parkinson’s made a great deal of effort to give lectures, leading to sporadic narratives.
The patients with Lewy body dementia deliver remarkable performance, with severe disturbances, even when using discourse basics for narrative production. In patients with DLB, dementia occurs at a higher level when producing individual words or generating sentences. They have considerable difficulties in relating successive events to each other; in other words, they are not able to relate story elements. In addition, the patients’ poor performance in the overall and intrinsic coherence of the story is also quite evident, and, therefore, they are significantly unable to preserve the theme of the story. This difficulty in expressing the organization of storytelling is most evident throughout it. According to our study results, which were consistent with the findings from the only research conducted by Ash et al., there was significant mental deficiency in speech and language disorders in patients with Lewy body spectrum disorder, which was most evident in the two groups with dementia (7, 8).
Therefore, dementia is the major cause of these disorders, which results from atrophy and thinning of the cerebral cortex, especially in the frontal area of these patients. They have problems in narrating the story throughout the storytelling process, such as difficulties in communicating the story events, maintaining the story’s theme, establishing global and local connectedness, and retrieving vocabulary, which earlier studies had shown to impair the organization of narrative deficits. Control of executive skills is concerned (17-19).
The present study, together with the study by Ash et al. (7, 8), was the only study that demonstrated the role of executive skills in the narrative discipline deficits of patients with dementia (with Parkinson’s or Lewy’s) in the two groups. Patients with Lewy body dementia also have significantly poorer executive function than patients with dementia with PD. Since the present study only investigated the speech disorders in patients with LBSD, therefore, it was recommended that similar studies should be carried out in order to evaluate speech disorders in other patients with frontal cortical gray matter atrophy and to illustrate other aspects of these problems. It was also suggested that similar studies should be conducted to explore this spectrum of patients with dyslexia as well as to reveal the association of this disorder with other underlying diseases and help to alleviate it. These further studies may have facilitated overcoming the problems of this kind.
As for the study limitation, other subjective linguistic and communication characteristics were not considered in our study and, therefore, any generalization of our results should be made with caution. In this regard, it was recommended that further studies should be carried out by adopting more experimental samples as well as addressing cognitive aspects and advanced linguistic levels in a structured way.