1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD; OMIM ID: 173900) is a monogenic multi-systemic disorder characterized by the development of renal cysts and various extra-renal manifestations. It is the most common inherited renal disease with a prevalence of approximately 1 in 400 to 1 in 1000 live births in all races [1]. This disease accounts for more than 10% of all cases of end-stage renal diseases (ESRD) [2]. ADPKD is characterized by numerous enlarged fluid-filled epithelial cysts typically in both kidneys and in some cases in other organs. Now 2 causal genes, PKD1 (MIM 601313) and PKD2 (MIM 173910), have been identified for ADPKD that are located respectively on chromosome 16 (16p13.3, 46 exons) and chromosome 4 (4q21, 15 exons) [3]. The PKD1 gene mutated in 85% of all ADPKD cases. Polycystin-1 is a receptor protein for cell-cell/matrix interactions which plays crucial roles in the regulation of cell proliferation and apoptosis [4]. In the present study, we report a case of novel mutation discovered from the direct mutation screening of all exons in the PKD1 gene in one Iranian patient with ADPKD.
2. Case Presentation
The patient was a 15-year-old boy, the only child of consanguineous parents. There was no history of ADPKD in this family. On sonography of the abdomen of the patient, the liver was normal in size, but contained multiple simple cysts, the largest was 27 × 27 × 25 mm located in the right lobe. On sonography of the kidneys and bladder, both kidneys were normal in position and however enlarged in size. Ten milliliter of peripheral blood was collected in EDTA containing tube. DNA was extracted by the standard salting out protocol. PKD1 gene-specific PCR primers were synthesized for all exons (a list of the primers used is available on request). The primers for exon 30 amplification was F: 5’-CCATGTTCCCTGGGTCTCT-3’ and R: 5’-GCTGCTCTCTCAACAAGAGGA-3’ The PCR was conducted under the following conditions: 100 ng genomic DNA, 200 μM deoxyribonucleotide triphosphates (dNTPs), 1.5 mm MgCl, 2.5 units supertaq polymerase, and 25 pmol each primer. Amplification was carried out in 25 μL volumes and 35 cycles: 93ºC for 1 min, 60ºC for 30 seconds and 72ºC for 45 seconds. Direct sequencing of the exons was performed using the big dye terminator cycle sequencing ready reaction kit (Applied Biosystems) on an ABI Prism 3700 automated genetic analyzer (Applied Biosystems). The same PCR primers were used for sequencing reactions. Finally, the sequences were compared to the reported gene sequence using the BLASTN program.
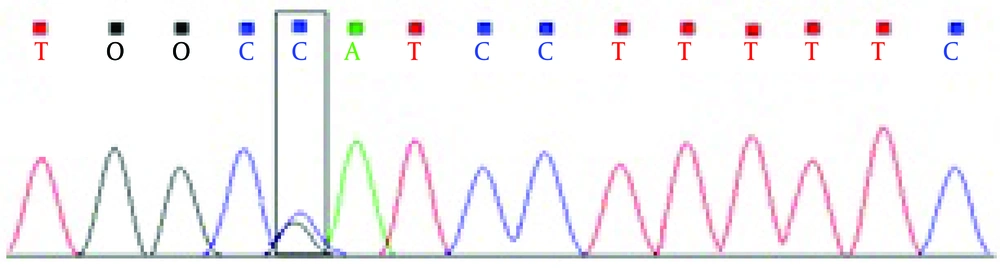
Direct sequencing analysis of the patient, after comparison with PKD1 reference sequence, demonstrated a sporadic heterozygous missense mutation CAT > GAT substitution in exon 30 of the PKD1 gene in the patient, which causes an amino acid exchange of histidine to argenine (Figure 1).
3. Discussion
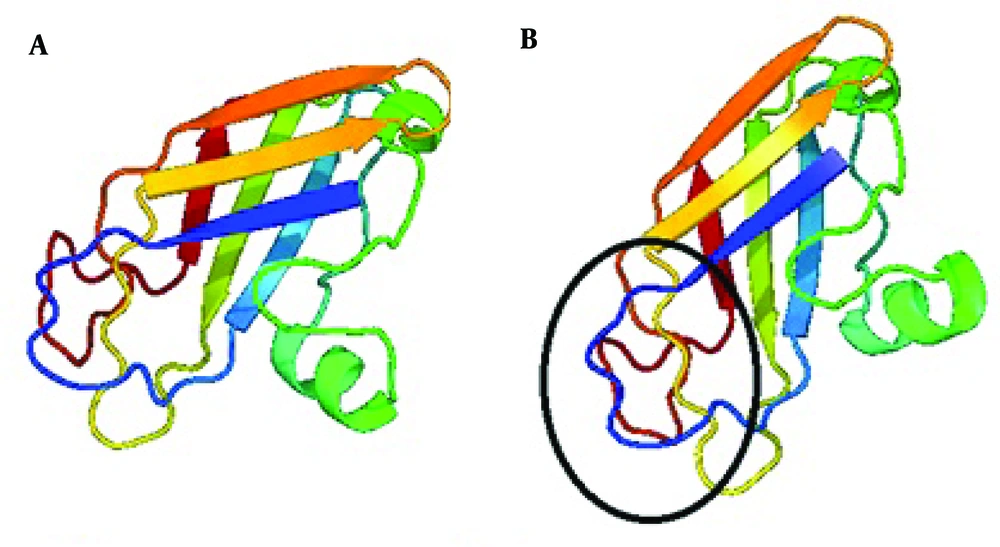
PKD1 gene mutations are considered as an important factor causing ADPKD. In 85-90% of patients mutations concern PKD1 gene and PKD2 in 10-15% of cases [5]. A large number of methods have been used to screen for mutations in ADPKD for research and clinical aims, but direct sequencing is still considered the method of choice [6]. We therefore performed systematic screening of the coding region of the PKD1 gene by PCR-direct sequencing method. The present study is the first description of the PKD1 variant in ADPKD patients living in Iran. We analyzed an affected person with polycystic kidney deficiency with PCR and direct sequencing of coding exons of the PKD1 gene. As a result we identified a genetic variant of PKD1 in ADPKD patient. It was a missense mutation CAT > GAT (H 3311R) located in the exon 30 of the PKD1 gene, that changed histidine to argenine in the transmembrane domain of the polycystin1 protein (Figure 2).
Polycystins are proteins that have been found to play important roles in cell to cell and cell to extracellular matrix interactions. Research into the function of these proteins has also revealed that they play a part in cilia action in the nephrons and calcium channeling [7].
Hydropathy plot and computer-assisted analyses identified 9 to 11 transmembrane regions from amino acid 3075 to 4104 with intervening intracellular and extracellular loops [8]. Our found mutation changes the transmembrane domain and disturbs the polycystin1 proper function. Nevertheless, it is a first step to future multicenter studies for better description of genetic variants in Iranian ADPKD patients.