1. Background
One of the most important stages of embryonic growth is organogenesis. The central nervous system (CNS) in humans originates in the middle of the third week in the form of a neural plate from the ectoderm, and after the edges of the plate fold, the neural folds come together in the middle line and form the neural tube. The head (crown) end of the tube is closed in about 25 days and the tail (rump) end is closed in 27 days. The result is the formation of the tube-shaped structure of the CNS of which its head end is wide (brain) and its tail end is long (spine). The growth of brain begins in the fifth week of pregnancy, and following it, the cerebral hemispheres are formed on the sides [1]. Organogenesis is intensively impacted by different environmental factors such as physical and chemical factors. Among the chemical factors; it is possible to refer to hormones and medicines. If a mother is not treated on time, it will be associated with the emergence of abnormality, acute consequences in the central neural system, preterm delivery, or increased embryo mortality. In addition, each factor under the impact of the dose consumed by the mother and the time of impact has different effects on the embryo [1-3]. Any kind of deficiency in the closing of the neural tube will bring about deficiencies in the embryo. Many embryonic abnormalities related to shortness in the neural tube such as spina bifida, encephalocele, and anencephaly has been proven in the past years [2, 4]. The growth of the central nervous system has a direct correlation with the growth and survival of an individual. A shortage in the evolution of neural cells leads to deficiencies in the natural performance of these cells from the viewpoint of discharge of cerebrospinal fluid and other materials necessary for the evolution of the central nervous system [5, 6]. The role of endocrine glands, including the thyroid gland, in the body’s metabolic activities is an undeniable reality. The effects of hyperthyroidism or hypothyroidism and related hormonal fluctuations can have an intensive impact on the mechanism of the body’s chemical interactions [7]. A delay in the growth of the brain and the cerebellum can happen because of hyperthyroidism or hypothyroidism [8, 9]. Any factor which causes a delay in the natural evolution of nervous cells will cause a disruption in the natural performance of nervous cells, and this deficiency can create many abnormalities in the nervous system, including microcephaly [10, 11]. The trend of brain evolution in rodents is a very good model for studying the action of thyroid hormones in the central nervous system [11, 12]. The best animal model of brain growth dependent on thyroid hormones is found in the infants of field rats [13]. Hyperthyroidism induction in animals is usually performed with the two methods of genetic induction and using medicine which can be planned based on the type and target of the study. An embryo’s brain growth in the beginning of pregnancy depends on the mother’s thyroid hormone. Non-treatment of hyperthyroidism in pregnant women causes a high risk for pregnancy poisoning, embryonic heart failure, lack of sufficient embryonic growth, preterm delivery, and embryonic death inside the womb. The greatest state of hyperthyroidism is in the form of Graves’ disease. Untreatedhyperthyroidism in pregnant mothers is associated with hyperthyroidism in embryos [14, 15].
2. Objectives
As thyroid hormones play key roles in the evolution of different systems, hyperthyroidism will create undeniable evolutionary deficiencies in the nervous system [16]. To date, the effects of hyperthyroidism on the evolution of the parietal lobe have not been studied from a histology point of view; the present research investigated this subject.
3. Methods
Approval for this experimental study was obtained from the Institutional Review Board, and all experiments were carried out in accordance with the guidelines of animal care and use of the ethics committee of Baqiyatallah University of Medical Sciences. Thirty mature female Sprague-Dawley field rats with a weight limit of 200 ± 10 grams were obtained from the Pasteur institute of Iran. The rats were placed in a controlled environment with a temperature of 23 ± 2°C, humidity of 50 ± 5%, and 12 hours of light and 12 hours of dark per day. To allow them to acclimate to the environment, all male and female rats were kept for two weeks separately in the animal house of Baqiyatallah University of Medical Sciences and were fed with standard food. The rats were randomly divided into three groups. The control group which received no materials remained intact. The sham group received a distilled water solution with common salt and polysorbate, and the experimental group received intraperitoneal injections of 0.5 mg/kg levothyroxine during a 10-day period. All rats were weighed and blood was taken was done through the sinuous inside of the eye orbit. Two weeks after the beginning of injections, blood was again taken from all groups. To determine the rates of T3, T4, and TSH hormones, the samples were sent to the endocrinology laboratory at Taleghani hospital. A gamma counter and R.I.A. kits were used to measure T4 and T3, and an IRMA kit was used to measure TSH.
After definite detection of hyperthyroidism in the rats of the experimental group, the rats in all groups were mated. Day zero of pregnancy was determined in the pregnant rats of the experimental group, and throughout the pregnancy, IP injections of T4 were made daily; the rats of the sham group received daily injections of distilled water solution containing polysorbate and Nacl.
At the end of day 20 of pregnancy, the pregnantrats were killed by inhalation of chloroform in a high dose. Initially, blood samples were taken from each mother rat and sent to the lab. Then the embryos were separated from inside the uterine horns, and the weight of the embryos and the crown-rump length (CRL) of the embryos were measured and registered. Finally, the embryos were placed inside a container containing 10% formalin for fixation. Five embryos out of the embryos of each pregnant mouse were randomly selected. After texture processing, sections measuring 5 microns thick were prepared, stained with the haematoxylin-Eosin (H and E) method, and evaluated by optical microscope and Motic software. The data was reviewed using the ANOVA method and Tukey test in SPSS 22 software. The information was presented in the form of mean ± SEM, and a difference in means was considered significant at the level of P < 0.05.
4. Results
During the tests, it became apparent that the embryos in both the control and sham groups were fully healthy and lacked any kind of morphological abnormality. The rate of T4 serum in the hyperthyroid mothers was about 265.8 ± 3.2 millimolar per liter, and in the control mothers it was 62.7 ± 2.3 millimolar per liter. The variables under examination in the mothers and embryos of the control and sham groups showed no significant difference in any case.
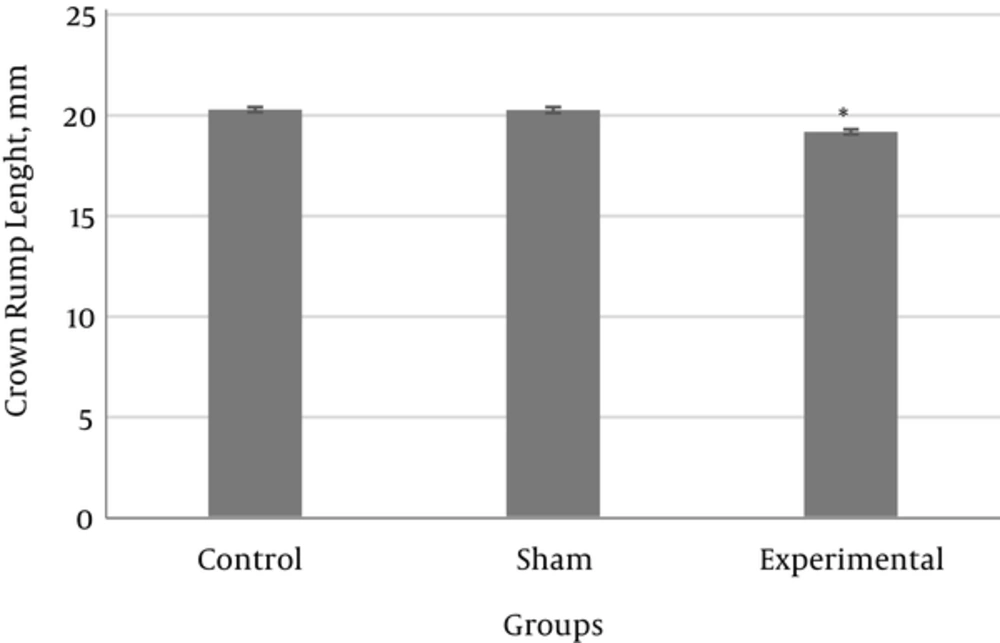
The results showed that the mean CRL length in the experimental group was significantly reduced compared with the control and sham groups (Figure 1).
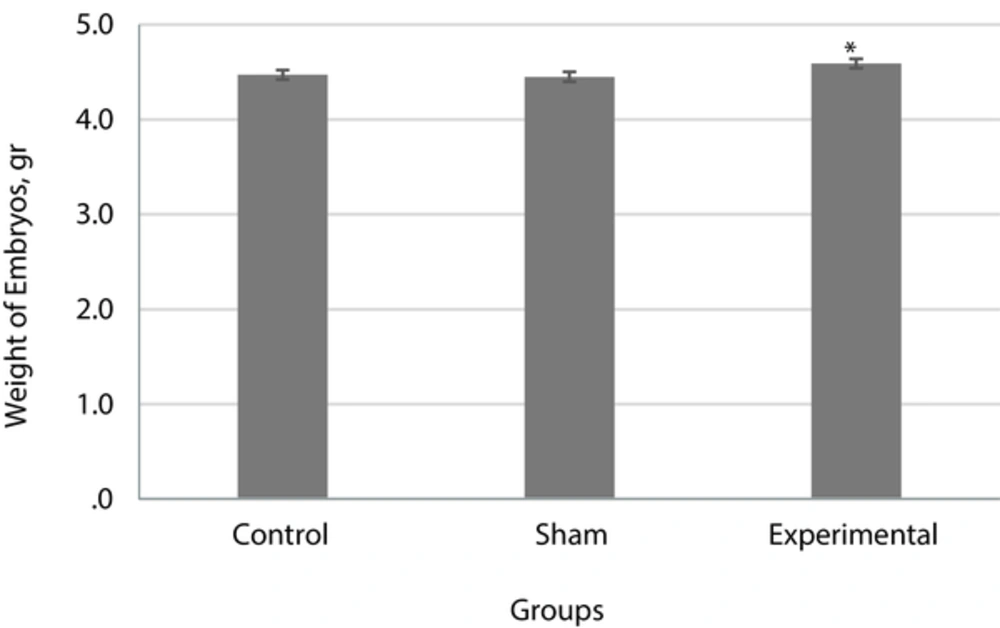
The mean weight of embryos in the experimental group showed no significant difference compared with the control and sham groups (P < 0.05) (Figure 2).
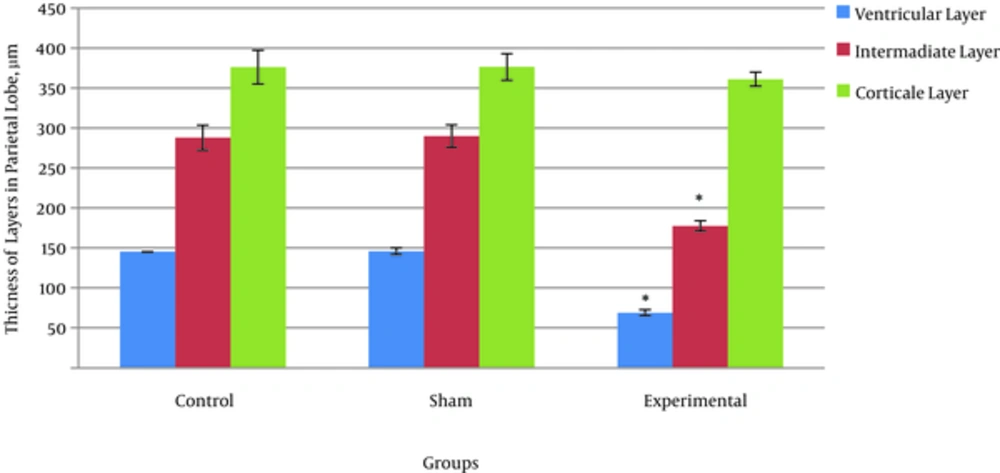
The results showed that the total thickness of the parietal cortex and the thickness of the cortical, intermediate, and ventricular layers in the experimental group as compared with the control and sham groups had a reduction; however, this reduction was insignificant only in the cortical layer (Figure 3).
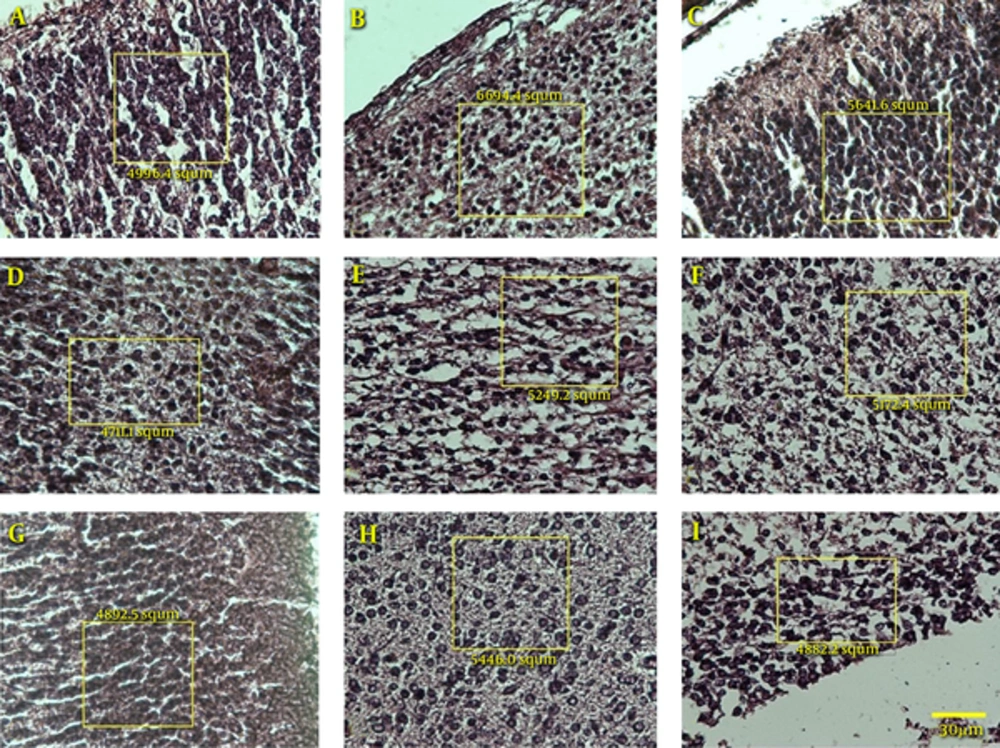
According to Figure 4, the number of cells in all three layers of the texture of the brain parietal cortex showed no significant difference between the control and sham groups. In all three layers, a difference is seen between the experimental group and the control and sham groups which indicates a reduction in the number of cells. However, only in the ventricular layer is there a significant difference between the experimental group and the control and sham groups (P < 0.05).
5. Discussion
Research has shown that, in addition to embryonic thyroid hormones, the maternal thyroid hormones are also necessary for the natural growth of the central nervous system before birth. A shortage of thyroid hormones during the pre-birth period will lead to irreversible damage to the brain and mental retardation [14]. Madeira et al. (1991) reviewed the impacts of hyperthyroidism on the granular layer of the dentate gyrus of the hippocampus in mature male and female rats. They concluded that a shortage of thyroid hormones causes a reduction of neuronal multiplication and increase of cell death [17]. Maternal hyperthyroidism can be a factor in increasedembryonicabortion and absorption, reduction of infant brain size and weight, and increasedembryonic mortality.
The rate of T4 serum in hyperthyroid mothers (experimental 265.8 ± 3.2) and in the control mothers (62.7 ± 2.3 nmol/L) had a significant difference. The research of Azizi et al. showed that the T4 and T3 serum levels were significantly higher in the group of hyperthyroid patients than in the control group [18]. The findings of the present study indicated a significant increase in the serum levels of T4 thyroid hormones, in agreement with Azizi et al. During pregnancy in humans and rats, the maternal thyroid hormones, in particular the Tiroxin hormone (T4), are transferred to the embryo through the placenta. The T4 hormone is changed into its active form, i.e. T3, after its transfer into the embryonic brain or the removal of iodine from it. If the rate of these hormones is at a natural level, the embryo develops naturally; a decrease or increase in the rate of thyroid hormones of the mother can be a factor of disruption of growth in embryos [19, 20]. The fluctuations related to each hormone can intensively influence the mechanism of body chemical interactions [21]. The mother’s thyroid hormones, particularly in the first half of pregnancy, are significantly important for the evolution of the embryo [22].
In the present research, the weight of embryos in the experimental group compared with those in the control and sham groups had a significant increase. These results are in agreement with the findings of Freidoun Azizi et al. in 2003 who showed that maternal hyperthyroidism causes the acceleration of osseous cells and increases the activity of osteoblast cells in embryos. It seems that the increase of embryonic weight in the experimental group of this study was due to the increased rate of osteoblast. The length of long bones and their mineralized part, in particular in the bones of upper organs in the embryos of the experimental group, showed that maternal hyperthyroidismand the passage of thyroid hormones through the placenta caused the acceleration of osseous growth and increase dosteoblast cells in embryos [18].
The results of the present research showed that maternal hyperthyroidism causes a significant reduction in CRL of the embryos in the experimental group compared with the control group. In astudy by Freidoun Azizi et al., the size of CRL between embryos of the experimental and control groupswas not significantly different, and these results are in agreement with the findings of the current study [18]. In the present research, the mother’s hyperthyroidismhad no impact on the weight and size of the placenta in the control and experimental groups. In the study of Freidoun Azizi et al., the weight and length of the placenta in embryos of the experimental and control groups did not show a significant difference, and these results are in agreement with the current findings [18]. It seems that the placenta in rats acts as an obstacle restricting the transfer of T3 [13].
The results obtained from the concerned parameters in this research indicated a kind of significant reduction in the thickness of the forming layers of the brain parietal cortex and a reduction in the number of their cells. In fact, it can be said that the impact of maternal hyperthyroidism induced with the maximum dose of levothyroxine led to a reduction in growth. The experimental groups on average showed a reduction in the thickness of layers and a reduction in the number of cells compared with the control group. The results of histomorphometry and cell counting in a study showed that the mother’s affliction with hyperthyroid disease during pregnancy controlled cell multiplication in the external granular layer of the cerebellum and diminished the neural migration in the inner layer towards the side of the cortical area [23, 24]. It seems that maternal hyperthyroidism also causes a delay to occur in embryonic brain growth, because the shortage of thyroid hormone in the embryotic period causes unnatural growth and a lack of distinction in cerebellum cells. This issue was also observed in rodents, i.e. the impact of the thyroid hormone on the trend of evolution of the cerebellum by two weeks after birth [20]. If the thyroid hormones do not discharge sufficiently in the embryonic period, the natural evolution of the cerebellum will face difficulty [25, 26].These results are in agreement with the findings of the current study.
5.1. Conclusion
The present study showed that maternal hyperthyroidism during pregnancy can have a negative impact on the evolution of the central nervous system and cause a reduction in growth of the CNS in the embryos of these mothers. Maternal hyperthyroidism leads to the emergence of many embryonic abnormalities and deficiencies. This study confirms well the impact of maternal hyperthyroidism on organogenesis, in particular in embryonic growth and evolution of the central nervous system.