1. Background
Secondary bacterial infections pose a considerable complication associated with viral respiratory infections, often leading to clinical worsening and, in severe instances, death. Historically, these infections have played a significant role in contributing to morbidity and mortality during post-influenza pandemics, seasonal influenza outbreaks, and other respiratory illnesses. The immune response activated by the virus causes damage to the airway epithelium and impairs its barrier function, which disrupts both innate and adaptive immune responses, thereby promoting the colonization of various bacterial pathogens (1-4).
Research has indicated that secondary infections during the COVID-19 pandemic could play a significant role in elevated mortality rates. Analysis of samples from individuals who succumbed to influenza revealed that a majority of fatalities were likely due to secondary bacterial pneumonia. During the 2009 influenza A pandemic, severe or lethal bacterial complications were observed in approximately 25% of cases, with a higher prevalence among adults and those hospitalized in the intensive care unit (ICU) (2, 5, 6).
Bacterial co-infection and superinfection in individuals diagnosed with COVID-19 raised significant concerns throughout the pandemic. Various studies have documented the incidence, categorization, and underlying causes of these co-infections and superinfections in hospitalized COVID-19 patients, with most of the existing data consolidated in two key studies. It is particularly noteworthy that the incidence of superinfections in ICUs reached its highest level, peaking at 41% (7, 8). The risk factors associated with co-bacterial infections in patients diagnosed with COVID-19 and influenza encompass several elements, including age, the classification of pneumonia, pre-existing medical conditions, admission to the ICU, the occurrence of invasive surgical procedures, duration of hospitalization, and the administration of hormone therapy (9). The predominant categories of infections identified were respiratory infections, particularly ventilator-associated pneumonia (VAP), which could be either primary or associated with central venous catheters. In patients diagnosed with VAP who are critically ill, there was a notable prevalence of gram-negative bacteria, encompassing both fermentative and non-fermentative strains (10-12). In the context of gram-positive bacteria, Staphylococcus aureus was identified in roughly 20% of all cases of superinfection. In contrast, among patients experiencing bacteremia, the predominant isolates were coagulase-negative Enterococcus and Staphylococcus species (13, 14).
The likelihood of developing pneumococcal pneumonia after an influenza virus infection may be diminished through pneumococcal vaccination in pediatric patients. A significant number of severe cases involving co-infection with influenza and S. aureus were observed in children without any pre-existing medical conditions. The occurrence of co-infection with influenza and methicillin-resistant Staphylococcus aureus (MRSA) appears to be associated with a personal history of MRSA-related skin infections or exposure to individuals with such infections (15-17).
2. Objectives
This study was conducted to assess the prevalence of bacterial pneumonia among children hospitalized with influenza and COVID-19 at Mofid Hospital between October and March 2023.
3. Methods
This cross-sectional study, based on hospital data, was carried out on children diagnosed with COVID-19 and/or influenza who were admitted to Mofid Hospital.
3.1. Eligibility Criteria
The inclusion criteria were a confirmed diagnosis of COVID-19 through a polymerase chain reaction (PCR) test or influenza based on a PCR test from a nasopharyngeal swab sample, with patients referred to Mofid Children Hospital between October and March 2023. The sole exclusion criterion was the absence of consent to participate in the study.
3.2. Data Collection
Data collected included patient demographics such as age, gender, and weight, as well as clinical symptoms, pneumonia severity, recent attendance at kindergarten or school, history of prior hospitalization for pneumonia, any underlying health conditions, use of oral antibiotics in the preceding month, imaging results indicating lung involvement, organisms cultured from blood, pleural fluid, and tracheal tube secretions, peripheral blood leukocytosis, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, complications arising from pneumonia during treatment, the necessity for admission to pediatric intensive care units (PICU), mechanical ventilation requirements, characteristics of aspirated pleural fluid, treatment with first-line antibiotics for pneumonia, treatment response after 48 hours of antibiotic initiation, duration of antibiotic therapy, length of hospital stay, and the sensitivity and resistance of organisms to antibiotics as determined by antibiogram. Subsequently, the study examined the prevalence of bacterial pneumonia among patients with influenza and COVID-19 infections.
Bacterial pneumonia was described as an inflammation of the lung parenchyma based on the following six findings (Nelson guidelines for the treatment of community-acquired pneumonia in children) (18): A diagnosis was considered when two or more of the following six criteria were present.
1. Clinical symptoms consistent with pneumonia (fever and chills, chest pain, dyspnea, etc.).
2. Imaging findings in chest X-ray or CT scan of the lung (lobar or segmental, pleural effusion, empyema in favor of bacterial pneumonia, and interstitial or alveolar involvement in favor of viral pneumonia).
3. Initial laboratory findings including the number of peripheral blood leukocytes and neutrophil percentage, CRP, ESR, and blood culture (the presence of lymphopenia, low CRP, and low ESR in favor of viral infection; peripheral blood leukocytosis, increased CRP, and ESR are in favor of bacterial pneumonia).
4. Lung auscultation manifestation (rales, fine crackles, or decreased sound in a part of the lungs).
5. Epidemiological findings (epidemic of specific viral infections at specific times, such as the epidemic of COVID-19 or influenza at a certain point in time).
6. Positive culture of the organism from the blood or pleural fluid sample or both (pneumococcus, staphylococcus, gram-negative bacteria, etc.).
All participants were selected prospectively based on predefined clinical and laboratory criteria documented in medical records. We employed clear inclusion/exclusion criteria and standardized data collection methods to minimize bias. The sample size represents the entire accessible population.
3.3. Determining the Severity of Pneumonia (19)
3.3.1. Mild
Tachypnea is defined by age-specific respiratory rates. In newborns under one month, a respiratory rate exceeding 60 breaths per minute is noted. For infants aged one month to one year, the threshold is more than 50 breaths per minute. In children aged 1 to 5 years, tachypnea is indicated by a rate above 40 breaths per minute, while in individuals older than 5 years, a rate exceeding 20 breaths per minute is classified as tachypnea. In cases of mild pneumonia, tachypnea is present, but other symptoms such as cyanosis and intercostal muscle retraction are absent.
3.3.2. Moderate
In moderate cases, tachypnea is observed alongside retraction of the intercostal, suprasternal, or subcostal muscles, depending on the child's age.
3.3.3. Severe
Severe cases are characterized by tachypnea accompanied by retraction, as well as episodes of apnea or cyanosis.
3.4. Statistical Analysis
Quantitative variables were described as mean and standard deviation, and qualitative variables were described as frequency and percentage. Fisher's exact test or chi-square was used to compare variables. All analyses were performed using SPSS version 26. A P-value of less than 0.05 was considered statistically significant.
3.5. Ethical Issues
This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (ethical approval code: IR.SBMU.MSP.REC.1402.443).
4. Results
In this descriptive cross-sectional study, a total of 155 hospitalized patients diagnosed with secondary bacterial pneumonia were enrolled from October 1 to March 2023. Among these 155 hospitalized children, who were admitted primarily due to viral symptoms, 32 patients met the criteria for secondary bacterial pneumonia based on four specific factors: Clinical symptoms, radiological findings, primary laboratory results (including peripheral blood leukocyte count, CRP, and ESR), and auscultation findings. All 155 patients underwent testing for influenza and COVID-19, with the exception of two patients who tested positive for influenza via PCR and were subsequently included in the group of 32 patients.
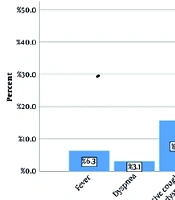
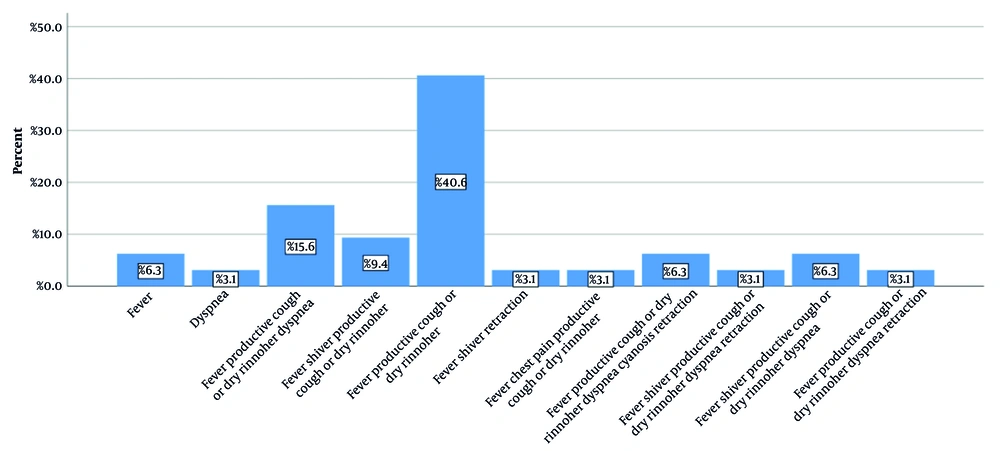
The mean age of the 32 patients with secondary bacterial pneumonia was 5.21 ± 3.06 years, with the youngest being 1 year old and the oldest 13 years old. Sixteen (50%) patients were boys and sixteen (50%) were girls. Regarding the mean anthropometric findings, the mean weight was 18.58 ± 8.61 kg, the mean height was 106.03 ± 23.69 cm, and the Body Mass Index (BMI) Z-score was normal (-1.88 to 0.54 for boys and -1.88 to 0.66 for girls). Thirteen patients (40.6%) attended school or kindergarten, while nineteen patients (59.4%) did not. In Figure 1, the clinical symptoms of the patients are shown separately. As can be seen, fever, nasal discharge, and dry cough were the most common symptoms, observed in 13 patients (40.6%).
The previous records of the patients were checked, and their information is given in Table 1. None of the patients had a history of vaccination against influenza.
| Variables | Values |
|---|---|
| Prior hospital admission | |
| No | 26 (81.3) |
| Yes | 6 (18.7) |
| Past medical history | |
| No | 29 (90.7) |
| Cerebral palsy | 1 (3.1) |
| Immunity deficiency | 1 (3.1) |
| Kartagener syndrome | 1 (3.1) |
| Recent antibiotic intake | |
| No | 19 (59.4) |
| Yes | 13 (40.6) |
a Values are expressed as No. (%).
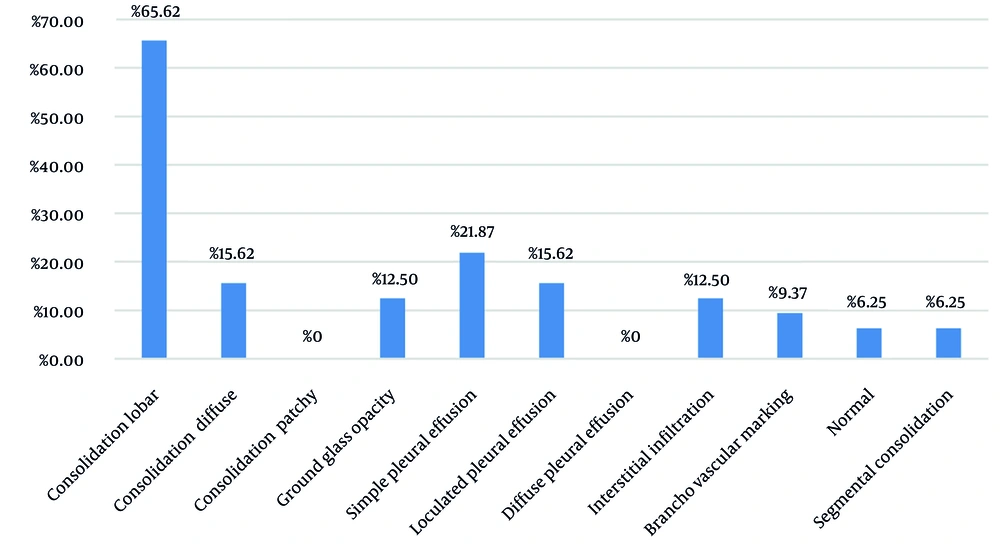
An analysis was conducted on the severity of bacterial pneumonia in the affected pediatric population. The findings revealed that 3 patients (9.4%) presented with mild pneumonia, 18 patients (56.3%) exhibited moderate pneumonia, and 11 patients (34.4%) were diagnosed with severe pneumonia. The imaging results for these patients are illustrated in Figure 2. Notably, lobar pneumonia was identified in 65.62% of the cases.
Laboratory results indicated that 15 patients (46.9%) exhibited leukocytosis, while all patients presented with negative blood cultures. The study also examined complications related to secondary bacterial pneumonia, the need for mechanical ventilation, and hospitalization in the PICU. The outcomes of these investigations are detailed in Table 2.
| Variables | Values |
|---|---|
| Complication of pneumonia | |
| None | 20 (62.5) |
| Pneumothorax | 3 (9.3) |
| Empyema | 2 (6.3) |
| Plural effusion | 1 (3.1) |
| Chest tube | 3 (9.4) |
| Pneumothorax, empyema | 2 (6.3) |
| Plural effusion, chest tube | 1 (3.1) |
| PICU admission | |
| No | 25 (78.1) |
| Yes | 7 (21.9) |
| Mechanical ventilation | |
| No | 30 (93.8) |
| Yes | 2 (6.2) |
Abbreviation: PICU, pediatric intensive care unit.
a Values are expressed as No. (%).
The mean hospitalization duration of the patients was 11.34 ± 8.63 days, and the mean length of treatment was 11.50 ± 7.90 days. The type of prescribed first-line antibiotics and the results of treatment with them were investigated. Information related to the type of antibiotics was evaluated. In terms of antibiotics, vancomycin was prescribed more than other antibiotics (71.8%). Next, meropenem was prescribed in 50% of cases, and ceftriaxone in 43.6% of patients. The combination of meropenem and vancomycin was prescribed in 46.9% of patients, and the combination of ceftriaxone and clindamycin was prescribed in 24.9% of patients. Out of 32 patients, 30 had a good response to antibiotics, and only 2 patients did not respond well to vancomycin, meropenem, ceftriaxone, and clindamycin, for which colistin and linezolid were prescribed, and they responded.
Finally, the relationship between secondary bacterial pneumonia severity with complications, hospitalization in PICU, and the need for mechanical ventilation was measured. It was found that the severity of secondary bacterial pneumonia is associated with hospitalization in PICU and the occurrence of complications (P < 0.05). However, there was no association between the severity of pneumonia and the need for mechanical ventilation (P = 0.131, Table 3).
The mean ± SD of ESR (mm/hr) and CRP (mg/L) were 58.47 ± 38.963 and 35.50 ± 21.466, respectively.
| Variables | Severity of Pneumonia | P-Value c | ||
|---|---|---|---|---|
| Mild (N = 3) | Moderate (N = 18) | Severe (N = 11) | ||
| Complication of pneumonia | - | 2 (11.1) | 10 (90.9) | 0.02 |
| PICU admission | - | 1 (14.29) | 6 (85.71) | < 0.001 |
| Need to mechanical ventilation | - | - | 2 (100) | 0.13 |
Abbreviation: PICU, pediatric intensive care unit.
a Values are expressed as No. (%).
b There was no mortality in our case.
c Fisher exact test or chi-square.
5. Discussion
In the present investigation, we sought to assess the prevalence of secondary bacterial pneumonia among pediatric patients in a tertiary care facility located in Tehran. Our findings indicated that out of 155 pediatric patients admitted for COVID-19 or influenza, 32 (20.64%) were diagnosed with secondary bacterial pneumonia. Respiratory viral infections, such as influenza and COVID-19, often result in secondary infections that can worsen disease severity and elevate mortality rates among those affected (20). This issue is particularly pronounced in influenza cases, as demonstrated by a recent meta-analysis of 27 studies, which reported an overall bacterial coinfection incidence of 23%.
The most commonly identified pathogens in these coinfections were Streptococcus pneumoniae, which accounted for 35% of cases, and S. aureus, which represented 28% of the coinfections, with significant variability noted across various studies (21). The incidence rates of secondary infections in patients diagnosed with COVID-19 have been reported to range from 5% to 100%. In critically ill patients, respiratory and bloodstream infections were found to be the primary sites of infection (22-32). In our study, we determined that the incidence rate of bacterial pneumonia was 20.64%, and notably, there were no fatalities among our patients. All blood cultures from the patients returned negative results, suggesting that the respiratory system was the main site of bacterial infections in our research.
Shafran et al. assessed the influence of secondary bacterial infections on the clinical progression and mortality rates of patients with COVID-19 in comparison to those with influenza. The findings indicated that patients with COVID-19 had a higher incidence of bacterial infections, with rates of 12.6%, compared to 8.7% in influenza patients. The presence of secondary infections was linked to an increased mortality risk in both cohorts, with a 2.7-fold increase (1.22 - 5.83) for COVID-19 patients and a 3.09-fold increase (1.11 - 7.38) for those with influenza. Mortality rates were significantly higher in COVID-19 patients when adjusted for age and clinical factors, whereas this adjustment did not yield significant results for influenza patients. The findings suggest that secondary bacterial infections represent a critical complication that leads to poorer outcomes in individuals with COVID-19 compared to those with influenza. Therefore, vigilant monitoring and timely antibiotic intervention may prove beneficial for these patients (33).
In the current study, we did not compare the clinical data of COVID-19 with influenza patients, which was one of the differences between the current study and Shafran et al.'s study. Although we did not find any mortality in our case, we found that antibiotic therapy helps to manage patients with secondary bacterial infection (33). It should be noted that antibiotic resistance should be considered in these patients because we found that 2 out of 32 (6.25%) of our patients were resistant to common first-line antibiotics, and we treated them by adding colistin and linezolid.
In a cohort of nineteen COVID-19 patients, Sharifipour et al. (23) reported that the mean duration of stay in the ICU was around 15 days. All patients tested positive for bacterial infections, with 17 strains of Acinetobacter baumannii (90%) and 2 strains of S. aureus (10%) identified. No variations in bacterial species were observed across different sampling points. All 17 strains of A. baumannii exhibited resistance to the antibiotics tested. Notably, no strains of A. baumannii producing metallo-beta-lactamases were detected. Among the S. aureus isolates, one was identified as methicillin-resistant and was obtained from the patient who passed away, whereas another strain was found to be methicillin-sensitive and susceptible to the antibiotics tested. This study highlights the significant risk of superinfection in COVID-19 patients associated with A. baumannii and S. aureus, underscoring the necessity of monitoring bacterial co-infections in critically ill COVID-19 patients.
Evidence reported that clinical and laboratory parameters in children diagnosed with COVID-19 infection can be used for the prediction of the severity of COVID-19 disease (34). Beliavsky et al. indicated that secondary bacterial infections following COVID-19 are linked to prolonged hospital and ICU stays, as well as an increased requirement for mechanical ventilation. They noted that the incidence of secondary bacterial infections in COVID-19 patients was 55% (35). In contrast, our study revealed an incidence of secondary bacterial pneumonia at 20.64%, which is significantly lower than the figure reported by Beliavsky et al. Our findings suggest that as the severity of secondary bacterial pneumonia increases, both the complications associated with pneumonia and the duration of PICU admission also rise; however, no association was found between the severity of pneumonia and the necessity for mechanical ventilation. In our study, the sample size in the subgroup of patients who need mechanical ventilation is limited, so the results are limited in generalizability (35).
Lai et al. reported on a cohort of 161 children diagnosed with COVID-19 who were hospitalized, among whom 24 exhibited bacterial coinfections, representing 14.9% of the total. The cohort with bacterial coinfections demonstrated increased white blood cell (WBC) counts. Additionally, this group had a higher proportion of patients requiring high-flow nasal cannula oxygen. The length of hospitalization and the duration of stay in the PICU were significantly prolonged for children with COVID-19 and bacterial coinfections. Importantly, there were no fatalities recorded. Identified risk factors for bacterial coinfections in the context of COVID-19 included abdominal pain, diarrhea, and neurological symptoms (36).
In the current study, we found that the rate of secondary bacterial pneumonia was 20.64%, which was relatively similar to the report by Lai et al. We demonstrated that the severity of bacterial pneumonia was associated with longer PICU stays and the complications of secondary bacterial infection, which was relatively similar to Lai et al.'s findings (36). We did not assess the risk factors for the presence of secondary bacterial infection, but we found that the most common clinical manifestations were fever, cough, and dyspnea, which were not the clinical manifestations Lai et al. mentioned. This finding was different between the two studies, and the causes of this difference should be evaluated in further studies (36). Similar to Lai et al.’s study, we had no deaths in our cases. In terms of laboratory studies, we found that inflammatory markers like WBC and ESR were elevated during the assessment, which was similar to Lai et al.’s study (36).
Alqahtani et al. conducted a study to evaluate the impact of secondary bacterial infections on the admission of COVID-19 patients to ICUs in Saudi Arabia. Their findings indicated that the duration of hospitalization for patients suffering from both COVID-19 and bacterial infections was considerably longer compared to those without bacterial infections. Furthermore, the mortality rates were notably elevated among patients with secondary bacterial infections (37).
In our current investigation, we observed that the length of stay in the PICU increased in correlation with the severity of pneumonia caused by secondary bacterial infections; however, no fatalities were recorded in our cases. This suggests that prompt intervention for patients experiencing secondary bacterial infections following viral infections may contribute to a reduction in mortality rates.
In our study, the mean ESR was 58.47 mm/hr (SD = 38.963) and the mean CRP was 35.50 mg/L (SD = 21.466). Although our study did not include a non-bacterial pneumonia group for direct comparison, it is well-established in the literature that CRP levels are generally higher in bacterial pneumonia than in non-bacterial forms (38). However, ESR levels do not have optimal sensitivity for differentiating between bacterial and non-bacterial pneumonia (39, 40).
Clinical studies conducted in China reveal that patients diagnosed with COVID-19 are often prescribed antibiotics, including azithromycin, moxifloxacin, ceftriaxone, vancomycin, and cefepime, as a preventive strategy against hospital-acquired infections. This practice is categorized as prophylactic. In cases where bacterial infections occur despite the use of antibiotic prophylaxis, particularly those involving drug-resistant strains, alternative combinations of the same antibiotics are utilized for therapeutic purposes. Both prophylactic and therapeutic approaches employ the same classes and dosages of antibiotics; however, many health organizations and governmental authorities globally advise against prophylactic use. This recommendation is primarily due to the increasing prevalence of antibiotic resistance, which is associated with the overuse and inappropriate application of these drugs. Additionally, the risk of superinfection with multidrug-resistant (MDR) bacteria complicates the treatment of critically ill COVID-19 patients in ICUs (41-43).
In our cases, we administered vancomycin, meropenem, ceftriaxone, and clindamycin, resulting in a favorable treatment response. Only two patients did not respond adequately; however, after incorporating colistin and linezolid into their treatment regimen, they achieved full recovery.
Given that only 32 patients had secondary bacterial pneumonia and this sample size cannot be generalized to the entire population, the results should not be interpreted as a general rule. The sample selected was only hospital patients, and in the community, healthier people naturally become infected with influenza or COVID-19. Therefore, only referrals are not the decision-making criteria, and the written interpretations should be used with caution. A more generalizable conclusion may be presented after the publication of related meta-analyses in this field.
While our study focused on describing secondary bacterial pneumonia in pediatric patients with influenza and COVID-19, we acknowledge that comparing patients with and without secondary bacterial pneumonia using advanced statistical models could have provided further insight into potential risk factors. However, due to the relatively small number of cases, applying such models would likely result in overfitting. We suggest that future studies with larger sample sizes explore these associations using more robust multivariable approaches.
5.1. Conclusions
The analysis showed a high rate of secondary bacterial pneumonia in children recovering from COVID-19 and influenza, with pneumonia severity linked to ICU admissions and complications. Common symptoms included fever, cough, and breathing difficulty. Most patients responded well to first-line antibiotics, and no deaths were reported. These findings highlight the importance of early recognition and empirical antibiotic treatment. Future research should focus on balanced viral groups, accurate microbiological confirmation, and comparative analysis.