1. Background
Coronaviruses comprise a large family of viruses responsible for illnesses ranging from the common cold to severe respiratory syndromes such as Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). In December 2019, a cluster of pneumonia cases with an unknown etiology was linked to a seafood market in Wuhan, China, marking the emergence of SARS-CoV-2, the causative agent of COVID-19. The disease manifests with respiratory symptoms, fever, cough, and dyspnea, which, in severe cases, can lead to acute respiratory distress syndrome (ARDS), kidney failure, and death (1-8).
The COVID-19 pandemic has had significant worldwide psychological, social, economic, and educational consequences. Reports indicate increased stress, anxiety, depression, social isolation, and domestic violence, alongside economic instability, job loss, and disruptions in education due to prolonged lockdowns (9-11). As of May 13, 2022, global statistics from the World Health Organization (WHO) recorded 517,648,631 confirmed cases and 6,261,708 deaths, with Iran reporting 7,227,043 cases and 141,201 deaths (12).
Vaccination remains the most effective and cost-efficient strategy for reducing COVID-19 morbidity and mortality. However, the pandemic has placed immense pressure on healthcare systems worldwide, particularly in diagnostics, drug supply, and treatment capacity. Economically, the crisis has led to reduced national productivity, slowed economic growth, and increased unemployment (13-15).
The COVID-19 viral genome was publicly released on January 11, 2020, enabling global vaccine development. Vaccines are critical in strengthening the immune system, preventing millions of deaths annually. Prophylactic vaccination remains the safest and most cost-effective strategy to prevent illness and death from COVID-19 and combat future variants (16).
The WHO has outlined ethical principles for vaccine distribution, including:
1. Maximizing benefits while minimizing harm — ensuring vaccines effectively reduce mortality, disease burden, and socio-economic consequences.
2. Prioritizing vulnerable populations — including healthcare workers, the elderly, and individuals with underlying medical conditions.
3. Ensuring equitable access — addressing barriers to vaccination due to socio-economic disparities (17).
Since February 18, 2021, at least seven different vaccines have been globally distributed, with over 200 vaccine candidates under development and more than 60 in clinical trials. These include Sinopharm and Sinovac (inactivated viruses), Pfizer and Moderna (mRNA vaccines), AstraZeneca, Johnson & Johnson, and Bharat (recombinant protein-based vaccines), and Sputnik V (viral vector-based vaccine) (18).
Despite global availability, vaccine distribution in Iran was shaped by government allocation policies, supply chain constraints, and priority group assignments. Regulatory approvals, manufacturing challenges, and logistical barriers further impacted vaccine availability. Healthcare workers were initially vaccinated with Sputnik V due to its early availability, whereas later phases introduced Sinopharm and AstraZeneca. Consequently, vaccine selection was not based on personal preference but rather dictated by national health regulations (19).
Studies suggest that vaccine acceptance in Iran was influenced by factors such as risk perception, trust in healthcare systems, and vaccination literacy. Additionally, demographic characteristics, prior exposure, and concerns regarding pandemic severity played significant roles in vaccine uptake (19). Understanding these determinants is essential for assessing vaccination trends and infection rates (20).
Despite extensive vaccination efforts, vaccine selection trends in Bam city have not been systematically studied. While existing research highlights factors such as health literacy, demographic characteristics, and risk perception, localized insights remain scarce.
2. Objectives
This study aims to analyze the determinants of vaccine choice and their association with COVID-19 infection rates among individuals visiting vaccination centers in Bam from the beginning of Iran’s vaccination rollout until November 2021.
3. Methods
The current cross-sectional descriptive-analytical study was conducted to investigate the factors related to the choice of vaccine among those referring to the vaccination centers of Bam city from the beginning of vaccination in Iran until the end of November 2021. The participants included the population referring to any of the corona vaccine injection centers in Bam city and received any of the available vaccines for the prevention of COVID-19 disease.
The tool used to collect information in this study is a researcher-made checklist. The questions of this checklist were prepared with the participation of the research team. This checklist included demographic information of the participants (age, gender, occupation, education level, history of COVID-19 disease, and underlying disease history) and questions regarding the history of infection, death of friends or family due to corona disease, type of vaccine, percentage of immunogenicity of vaccines, history of allergy to other vaccines or drugs, and clinical outcome after injection of the first dose of the vaccine.
The cluster sampling method was used in this study, where the 10 centers for corona vaccine injection in Bam city were considered as clusters. There were 10 operational vaccination centers in Bam city during the study period. All centers were included in the study to ensure comprehensive coverage of the population receiving COVID-19 vaccines. Since the total number of centers was 10, no selection process was required; instead, data collection was systematically conducted across all vaccination sites. This approach minimizes selection bias and ensures a representative sample from the entire city.
A specified sample size was determined for each cluster. Within the selected clusters, simple random sampling was employed to collect the desired variables. The sample size (1,100 participants) was determined using Cochran’s formula to ensure statistical validity. Since no prior data on vaccine effectiveness in this population was available, P = 0.5 was used for a conservative estimate. Based on a 95% confidence level (Z = 1.96) and a 3% margin of error (d = 0.03), the minimum required sample size was calculated using the formula:
Data were extracted from the Sib Vaccination Registry using a standardized checklist. The checklist was developed based on previous literature and the content of the study framework. As a researcher-designed tool, it was carefully constructed to align with the study objectives and relevant guidelines. For records with missing critical fields (e.g., vaccination date or demographic data), replacement participants were randomly selected from the same center's registry using a predefined algorithm to preserve population structure. The protocol was approved by the Bam University Ethics Committee (ethics code: IR.MUBAM.REC.1403.087), requiring verbal re-consent during follow-up contacts.
Immunogenicity percentages were estimated based on self-reported clinical outcomes (e.g., symptomatic infection) and verified through local health authority records. These values were aligned with efficacy rates published by the Iranian Ministry of Health for each vaccine type during the study period. The inclusion criteria consisted of all individuals aged 12 years and older who received a COVID-19 vaccine at one of Bam city's vaccination centers. However, a small subset of participants under 12 years of age was included in the dataset due to vaccination eligibility exceptions (e.g., clinical trial participation or special health conditions). Therefore, the demographic breakdown includes an "Under 12 years" age category to reflect these cases.
The exclusion criteria included people aged over or under 12 years who did not receive the vaccine for any reason, including age conditions, illness, complications, and severe sensitivity or apathy. The initial target sample size was 1,100, calculated based on the cluster sampling design. However, after data collection and quality control, 1,091 participants met the inclusion criteria and had complete datasets suitable for analysis. This minor reduction is due to incomplete records or non-responses, which were removed to maintain data integrity.
The collected data were analyzed using SPSS version 21 software and descriptive and inferential statistics. Quantitative information was reported in the form of mean and standard deviation using univariate and multivariate regression tests, and qualitative information was reported in the form of numbers, percentages, tables, and graphs. The significance level was considered 0.05 in all the tests.
4. Results
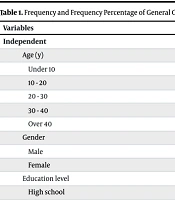
Table 1 presents the frequency and percentage of the demographic variables among the study participants.
| Variables | Values |
|---|---|
| Independent | |
| Age (y) | |
| Under 10 | 153 (14) |
| 10 - 20 | 195 (17.9) |
| 20 - 30 | 240 (22) |
| 30 - 40 | 211 (19.3) |
| Over 40 | 292 (26.8) |
| Gender | |
| Male | 552 (50.6) |
| Female | 539 (49.4) |
| Education level | |
| High school | 226 (20.7) |
| Diploma | 382 (35) |
| Associate degree | 154 (14.1) |
| Bachelor's degree and above | 329 (30.2) |
| Occupation | |
| Retired and disabled | 73 (6.7) |
| Employed in medical sciences | 125 (11.5) |
| Employed in other organizations | 146 (13.4) |
| Self-employed | 287 (26.3) |
| Student and University student | 180 (16.5) |
| Housewife | 280 (25.6) |
| Underlying disease | |
| Not have | 875 (80.1) |
| Heart disease and hypertension | 153 (14) |
| AIDS and MS | 5 (0.5) |
| Respiratory-pulmonary | 4 (0.4) |
| Cancer | 3 (0.3) |
| Psychological | 0 (0) |
| Diabetes | 51 (4.7) |
| Allergy to drugs or vaccines | |
| Yes | 43 (3.9) |
| No | 1048 (96.1) |
| Death of friends | |
| Yes | 311 (28.5) |
| No | 780 (71.5) |
| Complication | |
| No complication | 559 (51.3) |
| Body pain | 404 (37) |
| Fever | 106 (9.7) |
| dyspnea | 22 (2) |
| Dependent | |
| History of infection | |
| Yes | 523 (47.9) |
| No | 568 (52.1) |
| Type of vaccine | |
| Sinopharm | 400 (36.6) |
| AstraZeneca | 208 (19.1) |
| Sputnik | 122 (11.2) |
| Barkat | 136 (12.5) |
| Soberana | 165 (15.1) |
| Bharat | 60 (5.5) |
a Values are expressed as No. (%).
Table 1 presents the demographic distribution of participants. The largest age group was over 40 (26.8%), while the smallest was under 10 (14%). The gender distribution was nearly even, with males comprising 50.6% and females 49.4% of the sample. Regarding education, 35% of participants held a diploma, followed by 30.2% with a bachelor's degree or higher. In terms of occupation, the most common category was self-employment (26.3%), while retired and disabled individuals represented the smallest group (6.7%).
A notable 47.9% of participants reported a prior COVID-19 infection, while 80.1% had no underlying health conditions. Among those with preexisting conditions, heart disease and hypertension were most prevalent (14%). Only 3.9% reported allergies to drugs or vaccines. Regarding vaccine preference, Sinopharm was the most commonly administered vaccine (36.6%), followed by AstraZeneca (19.1%), Sputnik (11.2%), and others. Additionally, 28.5% of participants experienced the loss of friends or family members due to COVID-19.
Post-vaccination complications were reported by 48.8% of participants, with body pain being the most frequent symptom (37%), followed by fever (9.7%) and dyspnea (2%). The remaining 51.3% reported no complications.
4.1. Investigating the Simultaneous Effect of All Factors on the Choice of Vaccine Type
Multiple nominal logistic regression was used. This regression is applied in situations where the dependent variable is a nominal variable and the independent variables have different measurement levels (nominal, ordinal, and interval). A univariate analysis was conducted, and variables with a P-value less than 0.2 were included in the multivariate models. The results of the multiple nominal logistic regression are reflected in Table 2.
| Variables | Selected Vaccine Type Odds Ratio (P-Value) 95% CI for OR | P-Value | ||||
|---|---|---|---|---|---|---|
| AstraZeneca | Sputnik | Barkat | Soberana | Bharat | ||
| Age (y) | < 0.001 a | |||||
| Under 10 | 0.356 (0.072) (0.116 - 1.095) | 1.114 (0.891) (0.237 - 5.237) | 0.544 (0.321) (0.163 - 1.812) | 1.868 (0.262) (0.627 - 5.565) | 0.124 (0.120) (0.009 - 1.726) | |
| 10 - 20 | 0.658 (0.186) (0.354 - 1.224) | 0.669 (0.365) (0.280 - 1.596) | 0.888 (0.744) (0.436 - 1.809) | 0.140 (0.002) (0.039 - 0.496) | 0.310 (0.048) (0.097 - 0.992) | |
| 20 - 30 | 0.792 (0.447) (0.435 - 1.443) | 1.011 (0.980) (0.441 - 2.317) | 1.369 (0.355) (0.704 - 2.661) | 1.715 (0.158) (0.812 - 3.625) | 1.047 (0.931) (0.376 - 2.917) | |
| 30 - 40 | 0.850 (0.587) (0.474 - 1.526) | 1.019 (0.964) (0.443 - 2.344) | 1.122 (0.728) (0.587 - 2.145) | 1.929 (0.066) (0.958 - 3.886) | 1.868 (0.215) (0.695 - 5.021) | |
| Over 40 | Reference category | |||||
| Education level | < 0.001 a | |||||
| High school | 0.331 (0.003) (0.158 - 0.695) | 0.097 (0.002) (0.022 - 0.437) | 0.315 (0.006) (0.139 - 0.714) | 0.955 (0.913) (0.419 - 2.179) | 0.394 (0.319) (0.063 - 2.459) | |
| Diploma | 0.890 (0.645) (0.544 - 1.458) | 0.702 (0.322) (0.349 - 1.414) | 0.854 (0.564) (0.499 - 1.461) | 0.912 (0.801) (0.446 - 1.867) | 0.994 (0.990) (0.411 - 2.407) | |
| Associate degree | 1.180 (0.589) (0.674 - 2.154) | 1.217 (0.596) (0.589 - 2.516) | 0.468 (0.062) (0.211 - 1.040) | 3.720 (< 0.0001) (1.789 - 7.733) | 3.004 (0.011) (1.290 - 6.997) | |
| Bachelor's degree and above | Reference category | |||||
| Occupation | < 0.001 a | |||||
| Unemployed, disabled and retired | 1.016 (0.969) (0.462 - 2.233) | 1.901 (0.286) (0.584 - 6.191) | 1.302 (0.515) (0.588 - 2.883) | 1.012 (0.978) (0.428 - 2.395) | 0.309 (0.277) (0.037 - 2.565) | |
| Employed in medical sciences | 2.100 (0.095) (0.880 - 5.012) | 23.306 (< 0.001) (9.104 - 59.66) | 0.538 (0.375) (0.137 - 2.116) | 0.435 (0.446) (0.051 - 3.692) | 13.745 (< 0.001) (5.039 - 37.493) | |
| Employed in other organizations | 1.144 (0.676) (0.610 - 2.146) | 2.570 (0.031) (1.089 - 6.065) | 1.401 (0.312) (0.729 - 2.691) | 0.754 (0.495) (0.335 - 1.696) | 0.152 (0.019) (0.031 - 0.735) | |
| Self-employed | 1.114 (0.649) (0.700 - 1.774) | 0.891 (0.783) (0.392 - 2.025) | 0.713 (0.221) (0.415 - 1.226) | 0.903 (0.734) (0.502 - 1.624) | 0.449 (0.078) (0.184 - 1.093) | |
| Student or university student | 0.592 (0.267) (0.235 - 1.493) | 1.211 (0.773) (0.330 - 4.447) | 1.011 (0.983) (0.384 - 2.657) | 4.737 (0.004) (1.629 - 13.773) | 1.669 (0.477) (0.394 - 7.323) | |
| Housewife | Reference category | |||||
| Underlying disease | 0.001 a | |||||
| Not have | 2.238 (0.005) (1.280 - 3.914) | 2.687 (0.030) (1.102 - 6.549) | 1.516 (0.172) (0.834 - 2.755) | 0.559 (0.069) (0.299 - 1.047) | 3.410 (0.049) (1.004 - 11.577) | |
| Have | Reference category | |||||
| Allergy to drugs or vaccines | 0.074 | |||||
| Yes | 0.703 (0.574) (0.207 - 2.396) | 1.823 (0.366) (0.496 - 6.697) | 2.913 (0.027) (1.128 - 7.524) | 2.438 (0.062) (0.958 - 6.202) | 0.618 (0.674) (0.065 - 5.84) | |
| No | Reference category | |||||
| Death of friends | 0.021 a | |||||
| Yes | 0.722 (0.121) (0.478 - 1.090) | 0.610 (0.090) (0.345 - 1.079) | 0.695 (0.129) (0.434 - 1.112) | 1.098 (0.676) (0.709 - 1.699) | 1.792 (0.072) (0.95 - 3.38) | |
| No | Reference category | |||||
| History of infection | 0.002 a | |||||
| Yes | 0.540 (0.001) (0.371 - 0.786) | 0.584 (0.036) (0.353 - 0.966) | 0.740 (0.160) (0.487 - 1.125) | 0.862 (0.494) (0.564 - 1.319) | 1.590 (0.170) (0.820 - 3.08) | |
| No | Reference category | |||||
| Complications | < 0.001 a | |||||
| Weakness and body pain | 3.016 (< 0.001) (2.021 - 4.5) | 2.768 (< 0.001) (1.571 - 4.877) | 0.790 (0.311) (0.501 - 1.247) | 0.554 (0.010) (0.353 - 0.869) | 1.942 (0.051) (0.997 - 3.784) | |
| Fever | 9.484 (< 0.001) (4.775 - 18.834) | 9.484 (< 0.001) (4.165 - 21.598) | 0.926 (0.829) (0.345 - 2.489) | 0.386 (0.084) (0.131 - 1.138) | 2.268 (0.162) (0.721 - 7.136) | |
| Dyspnea | 3.962 (0.036) (1.096 - 14.32) | 9.071 (0.004) (1.992 - 41.309) | 2.282 (0.230) (0.593 - 8.789) | 0.398 (0.404) (0.046 - 3.473) | 1.411 (0.769) (0.142 - 13.98) | |
| No complication | Reference category | |||||
a Statistically significant.
In the multinomial logistic regression model, Sinopharm was designated as the reference category to assess the likelihood of selecting other vaccines. The results indicate that demographic characteristics, underlying health conditions, history of infection, and post-vaccination complications played a significant role in vaccine preference.
4.1.1. Education Level
Individuals with an Associate degree had 18% higher odds of selecting AstraZeneca compared to those with a Bachelor’s degree or higher (OR = 1.18). Conversely, individuals with high school education were 70% less likely (OR = 0.30) and those with a diploma were 12% less likely (OR = 0.88) to opt for AstraZeneca compared to the reference group. Associate degree holders had nearly four times higher odds (OR = 3.72) of selecting Soberana than highly educated individuals, while those with lower education levels were less likely to choose Soberana.
4.1.2. Underlying Disease and History of Infection
Individuals without underlying conditions had more than twice the odds (OR = 2.24) of selecting AstraZeneca compared to those with health conditions. Participants with a prior COVID-19 infection were 85% less likely (OR = 0.54) to opt for AstraZeneca. Similarly, individuals without underlying diseases were 2.69 times more likely to receive Sputnik compared to those with health conditions.
4.1.3. Complications and Vaccine Selection
Fever had the strongest association with vaccine choice — participants experiencing fever were nearly ten times more likely (OR = 9.49) to receive AstraZeneca. Those with weakness and body pain had 2.77 times higher odds of selecting Sputnik, while those experiencing dyspnea were nine times more likely (OR = 9.07) to opt for Sputnik. Participants with dyspnea were nearly three times more likely (OR = 2.91) to select Barkat, whereas individuals with fever or body pain showed lower odds of choosing Barkat.
4.1.4. Age and Vaccine Preference
Participants aged 10 - 20 years were more than seven times less likely (OR = 0.14) to select Soberana compared to those over 40 years old. Individuals aged 20 - 40 years had higher odds of choosing Bharat compared to the reference group (over 40 years old).
4.1.5. Occupation and Vaccine Selection
Medical professionals showed strong vaccine preferences — those employed in medical sciences were 23 times more likely to select Sputnik compared to housewives. Similarly, medical professionals were 13 times more likely to receive Bharat compared to Sinopharm.
4.2. Investigating the Simultaneous Effect of Variables on COVID-19 Disease
Table 3 presents the univariate and multivariate logistic regression results assessing the relationship between age, vaccine type, and the number of doses received with COVID-19 infection risk. Gender was excluded from the multivariate model due to non-significance in the univariate analysis.
| Variables | Univariate Logistic Regression | Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Coefficient Estimation | Odds Ratio (95% Confidence Interval) | P-Value | Coefficient Estimation | Odds Ratio (95% Confidence Interval) | P-Value | |
| Age (y) | ||||||
| 10 - 20 | -0.599 | 0.55 (0.22 - 1.39) | 0.205 | -0.921 | 0.40 (0.14 - 1.11) | 0.079 |
| 20 - 30 | 0.150 | 1.162 (0.64 - 2.13) | 0.626 | 0.13 | 1.14 (0.59 - 2.18) | 0.696 |
| 30 - 40 | -0.40 | 0.67 (0.36 - 1.26) | 0.214 | -0.37 | 0.69 (0.36 - 1.34) | 0.272 |
| Over 40 | Ref | Ref | ||||
| Gender | ||||||
| Male | -0.24 | 0.78 (0.47 - 1.28) | 0.333 | Omitted after univariate logistic regression | ||
| Female | Ref | - | ||||
| Vaccine dose | ||||||
| First | Ref | Ref | ||||
| Second | -1.47 | 0.23 (0.12 - 0.41) | < 0.0001 a | -1.82 | 0.16 (0.08 - 0.31) | < 0.0001 a |
| Third | -1.74 | 0.17 (0.07 - 0.39) | < 0.0001 a | -1.88 | 0.15 (0.06 - 0.36) | < 0.0001 a |
| Type of vaccine | ||||||
| Barkat | 0.95 | 2.60 (1.29 - 5.24) | 0.007 a | 0.87 | 2.38 (1.12 - 5.08) | 0.025 a |
| Pastokovic | -0.04 | 0.95 (0.38 - 2.39) | 0.921 | -0.038 | 0.96 (0.36 - 2.54) | 0.938 |
| Sputnik | 0.99 | 2.70 (1.31 - 5.57) | 0.007 a | 1.16 | 3.19 (1.47 - 6.95) | 0.003 a |
| AstraZeneca | -0.10 | 0.90 (0.36 - 2.26) | 0.831 | -0.36 | 0.70 (0.26 - 1.84) | 0.467 |
| Bharat | 0.56 | 1.75 (0.56 - 5.44) | 0.333 | 0.82 | 2.27 (0.71 - 7.24) | 0.165 |
| Sinopharm | Ref | Ref | ||||
a Statistically significant.
4.2.1. Vaccine Type and Infection Risk
Individuals who received AstraZeneca had a 30% lower likelihood (OR = 0.70, P > 0.05) of contracting COVID-19 compared to Sinopharm recipients, although this result did not reach statistical significance. In contrast, those vaccinated with Barkat (P < 0.05), Sputnik (P < 0.05), and Bharat (P > 0.05) had higher odds of infection relative to Sinopharm recipients, indicating that these vaccines may have conferred less protection in this sample.
4.2.2. Effect of Vaccine Dose on Infection Risk
Receiving a second or third dose was associated with an approximately sixfold reduction in the odds of contracting COVID-19, reinforcing the protective effect of booster doses in preventing infection.
5. Discussion
This study examined COVID-19 vaccine selection trends and infection risks, considering multiple demographic, clinical, and behavioral factors through multivariate analyses. Unlike previous studies focusing on single determinants, our approach provides a holistic perspective, highlighting the interactive effects of vaccination trends. However, several methodological biases should be considered when interpreting the results.
Our findings revealed that age, occupation, education level, prior infection history, underlying diseases, and post-vaccination complications significantly influenced vaccine choice. These results align with Sherman et al., who emphasized clinical outcomes and vaccine awareness as key determinants of acceptance (21). This mirrors findings from Molaeipour et al., who reported that vaccine acceptance in Iran was positively associated with education and occupation, and fluctuated with public trust and vaccine availability (22). Similarly, Omidvar and Firouzbakht found that risk perception, health literacy, and trust in the healthcare system were key predictors of vaccine uptake (19).
Our study found that education level significantly influenced vaccine selection. Individuals with an associate degree were nearly four times more likely to choose Soberana compared to those with a bachelor's degree or higher, while those with a high school education were significantly less likely to choose AstraZeneca or Barkat. This aligns with findings from Schafer et al., who reported that higher education was associated with increased vaccine acceptance among German university students, particularly those in health-related fields (23). Similarly, Joshi et al. emphasized that education enhances health literacy and trust in vaccine safety, thereby influencing vaccine preference (24).
Occupation also emerged as a strong predictor. Medical professionals were 23 times more likely to receive Sputnik and 13 times more likely to receive Bharat compared to housewives. This reflects early vaccine allocation policies in Iran, where healthcare workers were prioritized for Sputnik V. Gautier et al. found similar trends in France, where healthcare students in clinical training were significantly more likely to accept vaccination than those in non-clinical tracks (25). These findings suggest that occupational exposure and institutional mandates strongly shape vaccine uptake.
Participants without underlying diseases were more likely to choose AstraZeneca and Sputnik, possibly due to perceived lower risk of adverse events. This is consistent with Soleimanpour et al., who found that individuals with chronic conditions were more cautious and preferred inactivated vaccines like Sinopharm (20). However, a previous systematic review study found that factors influencing COVID-19 vaccine acceptance and hesitancy differ across regions, yet several universal elements — psychological, societal, and vaccine-specific — shape global acceptance (26).
In Iran, vaccine selection was largely dictated by availability rather than personal choice, particularly during the early rollout phase, when Sputnik V was allocated to healthcare workers before broader access to Sinopharm and AstraZeneca became available. Gender was not a significant factor in vaccine selection within our cohort, aligning with Grech et al. (27), which showed no substantial gender disparities in vaccine uptake. However, Luo et al. (28) reported higher vaccine acceptance among men, likely due to a higher occupational exposure risk. Bellon (29) noted women were more concerned about vaccine safety, which sometimes contributed to hesitancy. These discrepancies highlight the role of cultural differences, healthcare accessibility, and perceived risk in shaping vaccine preferences globally.
Notably, our study identified that individuals with fever or body pain were more likely to have received AstraZeneca or Sputnik, suggesting a link between reactogenicity and vaccine platform. This supports findings from Soheili et al., who reported higher systemic side effects with viral vector vaccines compared to inactivated ones (30).
Our finding that AstraZeneca recipients had lower odds of COVID-19 infection aligns with real-world evidence from the UK and Canada, where AstraZeneca showed 73 - 80% effectiveness against symptomatic disease and hospitalization after the first dose (31). In contrast, Sputnik, Barkat, and Bharat recipients showed a higher infection risk compared to Sinopharm recipients. This diverges from early clinical trial data, which reported efficacy rates above 90% for Sputnik V and 71% for Bharat (32).
One possible explanation for higher infection rates among Sputnik, Barkat, and Bharat recipients is the real-world effectiveness gap compared to controlled trials. Factors such as delayed administration of second doses, vaccine storage inconsistencies, and individual immune variability may affect real-world protection levels (33). Additionally, Sinopharm’s lower infection rates in our study may not necessarily indicate higher immunogenicity but rather reflect its predominant administration among older adults, who were more likely to adhere to post-vaccine precautions such as masking and limited exposure (34).
A key finding of our study was the protective effect of booster doses, with second and third doses reducing infection risk by nearly six times. This aligns with research showing that booster doses significantly enhance neutralizing antibody titers, providing longer-lasting immunity (35). The strong protective effect of receiving two or more doses is consistent with meta-analyses showing that second doses increase vaccine effectiveness from 71% to over 90% across platforms (30). However, our results differ from studies on mRNA booster effects, which showed stronger immune activation with mRNA vaccines compared to viral vector or inactivated virus-based vaccines (36). Given that mRNA vaccines were not widely administered in Iran, the durability of immunity observed in our cohort may differ from populations with higher mRNA booster uptake.
5.1. Conclusions
The study investigated how factors such as age, occupation, history of COVID-19 infection, underlying diseases, and post-vaccine complications influenced vaccine choice and infection risk among residents of Bam city, Iran. Among key findings, individuals who received the AstraZeneca vaccine had a 30% lower likelihood of contracting COVID-19 compared to those who received Sinopharm, although the result was not statistically significant. Conversely, those vaccinated with Sputnik, Barkat, and Bharat had higher odds of infection. Booster doses, especially second and third shots, significantly reduced the risk of contracting the virus by nearly six times.
Other influential determinants included education level, with individuals holding associate degrees more likely to opt for vaccines like Soberana, and medical professionals showing strong preferences for Sputnik and Bharat due to early allocation phases. For stronger future outcomes, the study recommends conducting serological testing to accurately assess immunity, designing longitudinal cohort studies to monitor long-term vaccine effectiveness, investigating behavioral causes of vaccine hesitancy, such as misinformation and healthcare trust, and implementing equitable vaccine distribution policies like subsidized programs and community outreach. These strategies aim to enhance scientific transparency, foster public trust, and improve booster dose adherence to mitigate hesitancy and strengthen pandemic resilience.
5.2. Limitations
While our models suggest strong associations, it is important to emphasize the limitations of a cross-sectional design, which prevents causal inference. Several confounding factors, including variations in exposure risk, preexisting immunity, and behavioral differences, may explain why some vaccines appeared to have different infection rates. Additional limitations include:
1. Recall bias: Self-reported data on infection history and vaccine side effects may be subject to memory distortions, affecting accuracy.
2. Selection bias: The cluster sampling method may have overrepresented specific demographic subgroups, limiting generalizability.
3. Absence of serological validation: The study did not measure antibody titers or cellular immune responses, restricting conclusions regarding immunogenicity differences. Future research should include serological testing for a more precise assessment.
4. Vaccine availability constraints: Early vaccination phases were dictated by government allocation policies, limiting the ability to analyze true vaccine preference in a free-choice scenario.