1. Background
Infertility is a significant aspect of reproductive health, affecting approximately 15% of couples, which translates to over 48 million couples worldwide (1). Female infertility accounts for 37% of all infertility cases (2) and is defined as the inability of a woman of reproductive age to conceive after one year of regular, unprotected intercourse (3). The predominant etiologies of infertility include ovulatory dysfunction, male factor infertility, and tubal pathology. Approximately 15% of cases remain idiopathic. In addition, lifestyle-related factors, such as cigarette smoking, have been identified as potential contributors to impaired fertility. Also, obesity exerts a detrimental effect on reproductive potential. Ovulatory disorders account for nearly 25% of infertility cases, with polycystic ovary syndrome diagnosed in approximately 70% of women presenting with anovulation (4). Despite extensive investigation, approximately 15 - 30% of infertility cases remain unexplained (3). Pathological agents, particularly viruses, have been implicated as major contributors to idiopathic infertility in several studies, with evidence suggesting that women are more susceptible to the effects of viral infections than men (5).
A specific subset of infertile patients experiences a disproportionately high rate of consecutive implantation failures during in vitro fertilization (IVF) treatment; in this context, recurrent implantation failure (RIF) may occur despite the transfer of morphologically high-quality embryos. This condition affects an estimated 10 - 15% of women undergoing IVF worldwide (6). Embryo-maternal communication is primarily mediated by various cytokines and chemokines, particularly interleukins (ILs), which are essential components of the uterine microenvironment (7, 8). Dysregulated interleukin (IL) production may impair implantation despite the transfer of morphologically high-quality embryos with strong developmental competence, thereby contributing to RIF (9, 10). Successful embryo implantation and pregnancy maintenance require a carefully balanced production of both pro- and anti-inflammatory cytokines. Interleukin-10 (IL-10) serves as a key immunoregulatory cytokine, suppressing T-cell proliferation and specifically inhibiting Th1-driven cellular immune responses (11). It also plays a critical role in regulating embryo-maternal tolerance, with its expression necessary for establishing a tolerant immunological environment within the endometrium (12).
Human papillomavirus (HPV) infection is a prevalent sexually transmitted infection, affecting approximately 50% of sexually active individuals at some stage of their lives (13). The HPV, a DNA virus infecting human epithelial cells, comprises nearly 200 genotypes, classified as low- or high-risk based on their ability to induce neoplastic changes (14, 15). Infertile women undergoing IVF show HPV-related abnormal cervical cytology or high-grade lesions at roughly twice the rate seen in the general population (16). Therefore, assessing the impact of HPV on IVF outcomes is crucial (17).
Human herpesvirus 6 (HHV-6) is a beta herpesvirus closely related to human cytomegalovirus (18). Originally classified into two variants, human herpesvirus 6A (HHV-6A) and human herpesvirus 6B (HHV-6B), recent findings suggest that each variant possesses distinct characteristics warranting classification as separate viral species (19). The HHV-6A/B DNA has been detected in genital tract secretions from approximately 4% of non-pregnant women and 2 - 18% of pregnant women, with higher viral loads observed in pregnant individuals (20). By disrupting endothelial cell function, HHV-6 can interfere with the establishment of a suitable uterine environment for implantation and fetal development, potentially leading to infertility and increased pregnancy loss (21). Despite significant advances in reproductive immunology and a better understanding of the immunological factors involved in the pathogenesis of RIF, further research is still needed in this area (22).
2. Objectives
The relationship between viral infections, specifically HPV and HHV-6, and RIF is an emerging field of interest, particularly concerning how these infections may influence IL-10 levels and overall fertility. Therefore, the present study aimed to investigate IL-10 expression levels and their relationship with the presence of HPV or HHV-6 viral genomes in women experiencing RIF.
3. Methods
3.1. Study Design
This cross-sectional study was conducted at the Infertility Center of Aban Hospital of Tehran. This study was approved by the ethics committee and the relevant code of ethics was obtained (IR.SBMU.MSP.REC.1401.059). All eligible patients received a detailed explanation regarding the objectives and protocol of the study, after which written informed consent was obtained. Ninety cases with RIF, defined as at least three unsuccessful embryo transfer cycles despite the transfer of high-quality embryos, were referred to the infertility clinic between September 2021 and March 2022. These patients were potential candidates for frozen-thawed embryo transfer (FET). Upon rigorous evaluation according to the inclusion and exclusion criteria, 20 patients fulfilled the conditions and were recruited. For aspiration of endometrial secretions, the patient was placed in a lithotomy position, a sterile speculum was placed inside the patient’s body, and the cervix was washed with a physiological syringe to prevent contamination with cervical mucus. Then, the Cook embryo transfer catheter (Guardia Embryo Transfer Catheters, Cook Medical, Bloomington, IN) was inserted into the uterus and 2 mL of endometrial secretions were aspirated using a syringe. The collected samples were frozen using the snap freezing method and transferred to a nitrogen tank. In the snap freezing method, collected samples must be transferred to a flask containing liquid nitrogen immediately after sampling.
3.2. Inclusion and Exclusion Criteria
The study included women diagnosed with RIF, defined as failure to achieve a clinical pregnancy after at least three embryo transfer cycles with good-quality embryos, ensuring that implantation failure was not due to poor embryo quality. All participants underwent frozen-thawed embryo transfer, were younger than 40 years, had a BMI < 30 kg/m2, and demonstrated a negative Pap smear result. Exclusion criteria were: (1) Endometritis; (2) polycystic ovary syndrome; (3) endometriosis; (4) uterine anatomical disorders; (5) use of intrauterine device (IUD) in the last three months; (6) use of hormonal contraception method in the last three months; (7) active uterine infection; (8) poor embryo quality; (9) untreated hydrosalpinx; (10) endocrine disorders (hypothyroidism, hyperprolactinemia, …); (11) adenomyosis; (12) intrauterine adhesions; (13) history of uterine incisions including myomectomies and cesarean sections; (14) uterine malformation; (15) the patient’s request to leave the study; (16) genetic disorder in the patient or her husband; (17) RIF patients on NSAIDs 10 days prior to the intervention or on corticosteroids over the past month; and (18) positive Pap smear test.
3.3. Multiplex Cytokine Immunoassay
We ordered the LEGENDplex™ Human IL-10 Capture Bead A10, 13X FlowAssay kit from BioLegend to analyze the IL-10 in endometrial secretion samples (biolegend.com/legendplex). A custom panel was designed for detection of IL-10. To collect samples from the frozen catheter tips, 1 mL of phosphate buffered saline was added to each frozen tip and allowed to reach room temperature (20 - 25°C) prior to use. All samples were analyzed according to the manufacturer’s instructions. The lyophilized standard panel was reconstituted with 250 μL assay buffer and 7 different standards were prepared according to the instructions. Then, 25 μL of each standard was added to the standard well and 25 μL of diluted secretion sample to the sample well. In the next step, 25 µL matrix was added to the standard wells, 25 µL assay buffer was added to the sample wells, and then 25 µL well-mixed beads were added to each well. The plate was shaken at 800 rpm for 2 hours at room temperature. After centrifugation, the supernatant was discarded and 200 μL of wash buffer was added to each well to wash the wells. In the next step, 25 μL of detection antibody was added to each well and shaken at 800 rpm for 1 hour. Next, 25 µL SA-PE was added directly to each well and shaken at 800 rpm for 30 minutes. After washing, the beads were suspended and read by flow cytometry (FACSLyric, BD, San Jose, CA, USA). Data were analyzed by the LEGENDplex data analysis software.
3.4. DNA Extraction
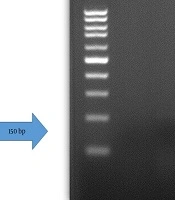
DNA extraction from endometrial secretion was carried out using the Sambio TM DNA Extraction kit (Sambio TM, Korea country) according to the kit protocol. Subsequently, all samples were quantified using a spectrophotometer and by reading the absorbance at (260/280 nm). Isolated DNA was kept at -20°C until analysis. The human beta-globin gene was used as an internal control to assess the adequacy of cellular DNA extraction. To verify the integrity of extracted DNA, a polymerase chain reaction (PCR) test was performed using primers specific for the human beta-globin gene. The primers used were: KM38 (5ʹTGGTCTCCTTAAACCTGTCTTG3ʹ) and PC03 (5ʹACACAACTGTGTTCACTAGC3ʹ) (Figure 1). The PCR was performed on all samples in addition to positive and negative controls (23, 24).
3.5. Nested-Polymerase Chain Reaction Assay
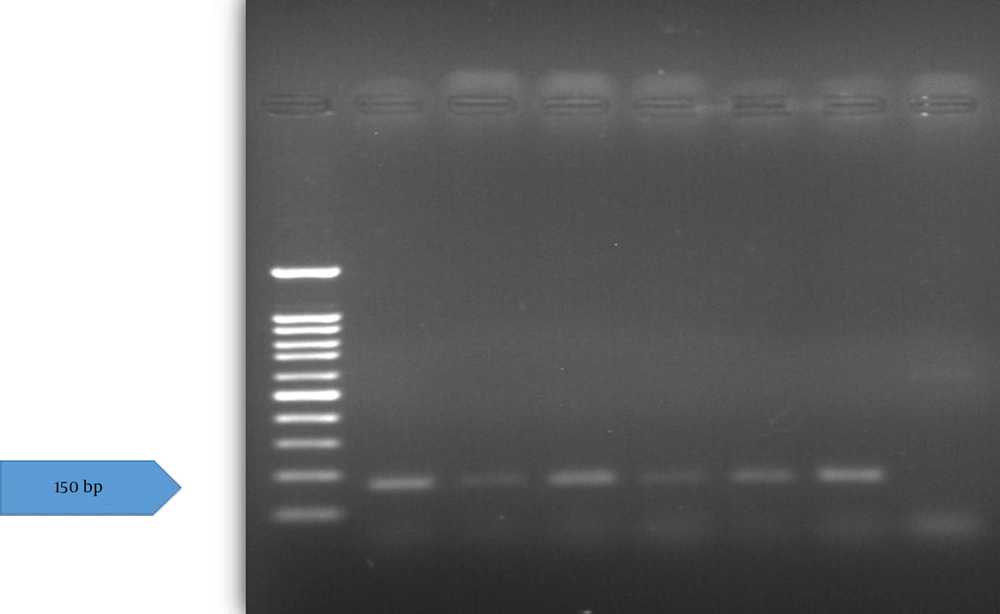
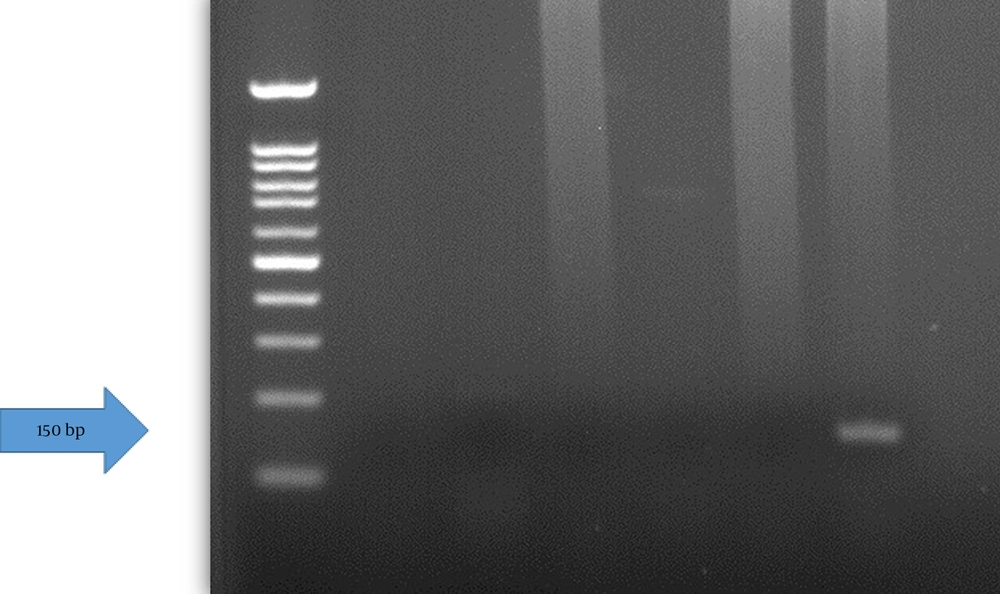
Following verification of the extracted DNA quality, the presence of the HPV genome was assessed using a nested-polymerase chain reaction (nested-PCR) assay. The PCR was first performed with MY09/11 primers (MY09: 5ʹCGTCCMARRGGAWACTGATC3ʹ, MY11: 5ʹGCMCAGGGWCATAAYAATGG3ʹ) and the second round used primers GP5+/6+(GP5+: 5ʹTTTGTTACTGTGGTAGATACTAC3ʹ, GP6+: 5ʹGAAAAATAAACTGTAAATCATATTC3ʹ) (Figure 2) (23). These primers target the HPV L1 gene. A 25 μL reaction mix contained 0.2 - 0.5 μg concentration of extracted DNA (or controls), 1 μM concentration of each forward and reverse primer, 12.5 μL of 2X master mix PCR (Fugen 2X Taq plus Master Mix), and distilled water to reach the final volume. The PCR was performed with an initial denaturation at 94°C (3 min), followed by 35 cycles of 94°C (30 s), annealing at 57°C (30 s), and 72°C (1 min), with a final extension at 72°C (10 min). The second round used the same program, except annealing at 54°C. Products (450 bp first round, 150 bp second round) were visualized on 1.5% agarose gel under UV light and documented with a gel documentation system.
3.6. Real-time Polymerase Chain Reaction
The HHV-6 RQ kit (Novin gene) provides a ready-to-use real time polymerase chain reaction (real-time PCR) assay for detection, differentiation, and quantitation of HHV-6A and HHV-6B. In addition to the number of tested samples, we considered 4 tubes for standards and one tube for negative control. 15 μL of HHV-6 mix and 10 μL of standards or extracted DNA or water was added to each tube. Then the tubes were placed in the Rotor-Gene 6000 machine.
3.7. Statistical Analysis
Data were analyzed using SPSS software version 27 (SPSS Inc., Chicago, IL, USA) and the chi-square test. P-values less than 0.05 were considered statistically significant.
4. Results
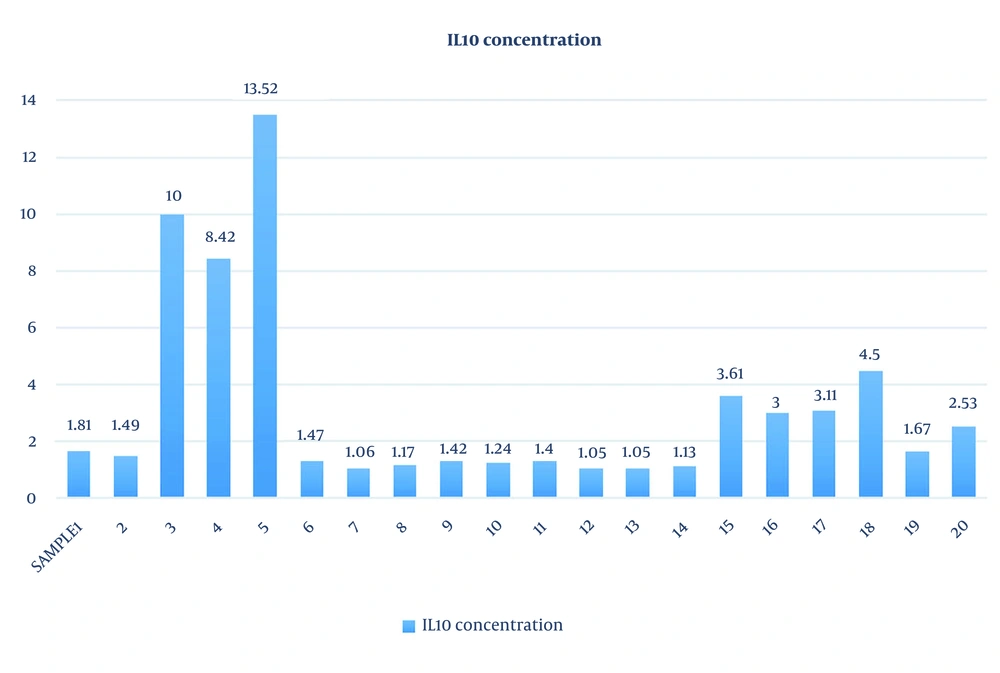
The study population age ranged from 32 to 38 years and the mean age was 34.95 ± 2.01 years. The mean duration of infertility among women was 5.9 ± 0.9 years. The IL-10 concentration was analyzed based on the flow cytometry method of the LEGENDplex™ kit. The range of IL-10 concentration was 1.05 to 13.52 (3.23 ± 3.4) (Table 1 and Figure 3). To verify the accuracy of extracted DNA, the beta-globin gene was tested for all samples, and except for the negative control, all samples were positive. Additionally, positive (HPV-positive sample taken from the Keyvan Virology Laboratory) and negative (distilled water) controls were used to confirm the HPV PCR steps. The HPV DNA was not detected in the samples. No significant relationship was observed between levels of IL-10 concentration and HPV DNA positive tests (P > 0.05). Among women with RIF, 5% (n = 1) demonstrated the presence of HHV-6A DNA, while 95% (n = 19) had no detectable viral transcripts. No significant association was observed between IL-10 concentration and the presence of HHV-6 in endometrial secretion samples (P > 0.05).
| Samples | Age (y) | BMI (Kg/m2) | IL-10 Concentration (pg/mL) | Number of Good Embryos | Duration of Infertility (y) | Abortion | HPV Status | HHV-6 Status |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 23 | 1.81 | 10 | 6 | Yes | Negative | Negative |
| 2 | 38 | 22 | 1.49 | 8 | 5 | No | Negative | Negative |
| 3 | 35 | 20 | 10 | 9 | 6 | No | Negative | Negative |
| 4 | 34 | 23 | 8.42 | 7 | 7 | No | Negative | Negative |
| 5 | 36 | 24 | 13.52 | 8 | 8 | No | Negative | Negative |
| 6 | 37 | 19 | 1.47 | 6 | 7 | No | Negative | Negative |
| 7 | 33 | 21 | 1.06 | 8 | 6 | No | Negative | Negative |
| 8 | 38 | 25 | 1.17 | 9 | 5 | No | Negative | Negative |
| 9 | 34 | 24 | 1.42 | 6 | 7 | No | Negative | Positive (HHV-6A) |
| 10 | 35 | 25 | 1.24 | 8 | 6 | No | Negative | Negative |
| 11 | 36 | 23 | 1.40 | 7 | 6 | No | Negative | Negative |
| 12 | 32 | 22 | 1.05 | 7 | 5 | No | Negative | Negative |
| 13 | 33 | 25 | 1.05 | 6 | 5 | No | Negative | Negative |
| 14 | 34 | 23 | 1.13 | 7 | 6 | No | Negative | Negative |
| 15 | 35 | 24 | 3.61 | 9 | 5 | No | Negative | Negative |
| 16 | 32 | 25 | 3.00 | 8 | 8 | No | Negative | Negative |
| 17 | 34 | 23 | 3.11 | 7 | 6 | No | Negative | Negative |
| 18 | 36 | 24 | 4.50 | 9 | 5 | No | Negative | Negative |
| 19 | 37 | 24 | 1.67 | 8 | 5 | No | Negative | Negative |
| 20 | 38 | 23 | 2.53 | 7 | 5 | No | Negative | Negative |
Abbreviations: IL-10, interlukin-10; HPV, human papillomavirus; HHV-6, human herpesvirus 6; HHV-6A, human herpesvirus 6A.
5. Discussion
This study provides new insights into the potential interplay between immune regulation and viral infection in women with RIF. Unlike most previous studies that examined either cytokine expression or viral infection independently, our work simultaneously evaluated IL-10 levels and the presence of HPV and HHV-6 DNA in endometrial secretions. This combined immunological and virological approach offers a more comprehensive understanding of endometrial factors potentially influencing implantation outcomes. Another unique aspect is the use of endometrial secretion samples rather than tissue biopsies or cervical swabs, providing a minimally invasive and physiologically relevant reflection of the uterine microenvironment at the implantation site. Furthermore, this is among the first studies in an Iranian population exploring the coexistence of viral infection and immune modulation in RIF.
Our findings revealed that women with RIF had lower IL-10 concentrations, aligning with previous research suggesting that impaired IL-10 production may contribute to a pro-inflammatory uterine environment, hindering successful implantation. An imbalance between pro-inflammatory and anti-inflammatory cytokines has been observed in women with RIF and chronic endometritis, who also exhibited significantly lower IL-10 levels in their endometrial tissue. Inagaki et al. (25) confirmed a statistically significant decrease in IL-10 in RIF patients through uterine cavity irrigation during the luteal phase, supporting findings by Banerjee et al. (26) on cytokine assessment during the window of implantation. Nevertheless, the role of IL-10 in RIF remains controversial, as other studies have reported non-significantly higher IL-10 levels in patients achieving successful pregnancies. Additionally, Liang et al. (27) reported no significant differences in serum IL-10 levels between RIF patients and controls prior to IVF or intra cytoplasmic sperm injection (ICSI) cycles. Patient-specific factors, including coexisting conditions such as endometriosis and the timing/type of sample collection within the menstrual cycle, further complicate the interpretation of IL-10 levels and contribute to inconsistencies in the literature.
In contrast to previous studies indicating that HPV infections are significantly associated with adverse effects on reproductive function (28, 29), this study found no evidence of HPV genome presence in any uterine endometrial secretion samples. While HPV infection can downregulate anti-inflammatory cytokines such as IL-10, which are crucial for maternal immune tolerance during implantation (30), our PCR-negative results suggest that HPV did not contribute to lower IL-10 concentrations in the studied population. In women with RIF, the presence of HPV may exacerbate the imbalance between pro-inflammatory and anti-inflammatory cytokines, creating a hostile uterine environment that hinders embryo implantation (31). Moreover, chronic inflammation associated with HPV infection could disrupt IL-10 signaling pathways, ultimately affecting implantation success (32). Therefore, it is essential to consider the potential role of HPV in impacting IL-10 production among women experiencing RIF. The HPV PCR negative result in addition to the negative Pap smear result as an inclusion criterion showed this virus has no effect on lower concentration of IL-10 among the studied population.
Although HPV DNA was not detected in any of the endometrial secretion samples, it is worth emphasizing that the use of endometrial samples — as opposed to vaginal samples — offers a more targeted approach for assessing viral presence in the upper genital tract, especially in infertile women. This method helps avoid potential false-positive results due to contamination from the lower reproductive tract (33).
The HHV-6A was detected in only one sample (5%), with no significant association observed between HHV-6 DNA positivity and lower IL-10 concentrations (P > 0.05). In women with RIF, HHV-6 infection may interfere with cytokine production, particularly inhibiting the synthesis of anti-inflammatory cytokines such as IL-10. Studies indicated that HHV-6 can disrupt the endometrial immune environment by promoting a Th1-dominant response, which enhances pro-inflammatory cytokines while suppressing IL-10 levels (34, 35). This dysregulation can create an unfavorable uterine milieu detrimental to embryo implantation (36). Furthermore, the persistence of HHV-6 in the endometrium has been linked to immune evasion mechanisms that exacerbate inflammatory responses, complicating reproductive outcomes (37). Notably, Marci et al. (38) reported for the first time that HHV-6 uterine infection might be a significant factor in the development of unexplained female infertility, with HHV-6 DNA detected in 43% of endometrial biopsies from primary unexplained infertile women compared to 0% in fertile controls. Additionally, Alazzam et al. (39) examined endometrial epithelial cells from 10 women with infertility and found that 40% of these cells were positive for HHV-6 DNA, while no viral DNA was detected in the endometrium of fertile women. When cultured, these endometrial epithelial cells produced early and late viral proteins, indicating the presence of an infectious virus. However, the small number and type of samples examined in this study may have led to different results from other studies.
One of the main limitations of this study was the small sample size (n = 20) and the absence of a control group consisting of women with successful IVF outcomes. While the inclusion of such a group would have strengthened the comparative aspect of the study, practical and ethical constraints — such as access to endometrial samples from women not experiencing implantation failure — prevented the recruitment of an appropriate control group. Additionally, despite negative Pap smear results for the samples, we performed HPV PCR testing, as PCR is more sensitive and a negative Pap smear does not necessarily indicate the absence of HPV. While Pap smears detect abnormal cell changes, HPV infection can exist without causing detectable alterations in the cells. Sample collection errors represent a common issue with Pap smears, as achieving adequate coverage can be challenging. Furthermore, optimal sample collection may be hindered by a lack of trained personnel (40). Furthermore, the low detection rates for HPV (0%) and HHV-6 (5%) substantially limit the statistical power and generalizability of the findings. These low rates may reflect inherent sampling and population constraints typical of RIF research. Therefore, the current results should be considered preliminary, and the observed trends — particularly for HHV-6 — require confirmation in studies with larger sample sizes and multicenter collaboration. Future studies with a larger sample size and matched control groups are warranted to validate and expand upon the present findings.
5.1. Conclusions
In this study, consistent with previous reports, low levels of IL-10 concentration were detected in the endometrial secretions samples of women with RIF, which may contribute to a pro-inflammatory uterine environment and hinder successful implantation. The dysregulation of IL-10 observed in our samples was not influenced by uterine HPV and/or HHV-6 infection. Future studies should involve a larger cohort of women with RIF and explore a broader range of viral pathogens to ascertain whether IL-10 levels are associated with specific viral infections.