Hidradenitis suppurativa (HS) is a chronic, relapsing, and debilitating inflammatory skin disorder characterized by the formation of recurrent nodules, abscesses, sinus tracts, and scarring. The disease primarily involves the folliculopilosebaceous unit in apocrine gland-bearing intertriginous areas, including the axillae, inguinal and anogenital regions, buttocks, and inframammary folds. Less frequently, lesions may occur on the posterior neck, inner thighs, and waistband areas (1). The global prevalence of HS is estimated at approximately 1%, with a female predominance. Onset typically occurs after puberty, most commonly during the second and third decades of life, suggesting a potential hormonal influence. The HS has been associated with other dermatological conditions such as acne vulgaris, pilonidal sinus, and hirsutism (1).
Clinically, HS begins with comedones, painful inflammatory nodules, and abscesses, which may rupture and discharge purulent material. Recurrent inflammation often results in the development of interconnected sinus tracts and extensive dermal fibrosis, contributing to chronicity and disfigurement. Secondary bacterial infection is common and may necessitate systemic antibiotic therapy. The disease course is variable, ranging from mild, localized involvement to severe, widespread, and mutilating forms (2).
The exact pathogenesis of HS remains incompletely understood; however, follicular occlusion, dysregulated immune responses, genetic predisposition, and microbial factors are implicated. Modifiable risk factors such as cigarette smoking and obesity are strongly correlated with both disease onset and progression, and are considered to worsen clinical severity (2).
These three statements represent only a subset of the 119 consensus recommendations outlined in the “North American Clinical Practice Guidelines for the Medical Management of HS in Special Patient Populations”, published in the Journal of the American Academy of Dermatology. This inaugural guideline provides expert-endorsed recommendations for the medical management of HS across seven clinically distinct populations: Pregnant and lactating patients, pediatric patients, individuals with malignancy, patients with tuberculosis (TB) infection, those with hepatitis B or C, and persons living with HIV (3).
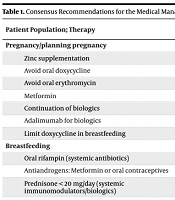
Among the 27 recommendations for patients with HS who are pregnant or planning pregnancy, the authors designated 8 as “strong” based on the quality of the supporting evidence, with the remaining recommendations rated as “conditional”. The strong recommendations encompass: Administration of zinc supplementation, avoiding oral doxycycline due to potential teratogenic risk, avoiding oral erythromycin because of the risk of hepatotoxicity and other adverse outcomes, use of metformin, continuation of biologic therapy, preferential use of adalimumab in patients requiring biologics, and limiting doxycycline in breastfeeding patients (3). Recommendations for other HS patient subgroups include the following.
Consensus Recommendations for Hidradenitis Suppurativa in Special Populations
The North American Clinical Practice Guidelines for the Medical Management of HS in Special Patient Populations provide a structured framework for managing HS in patients with unique clinical considerations. Across eight populations — pregnancy, breastfeeding, pediatrics, malignancy, TB infection, hepatitis B or C, and HIV — treatment recommendations are graded as “strong” or “conditional” depending on the strength of available evidence and clinical consensus.
- Strong recommendations indicate high-quality evidence and high confidence in the balance of benefits and risks.
- Conditional recommendations reflect situations where evidence is limited, benefits and risks may vary, or individualized clinical judgment is required.
The guidelines emphasize safety, disease control, and coordination with specialists (e.g., oncologists, hepatologists, infectious disease experts) when systemic therapies, biologics, or immunomodulators are indicated. Special considerations include teratogenicity, lactation safety, pediatric dosing, infection risk, and prior malignancy history (3). This information is summarized in Table 1. The table summarizes the strength of evidence-based recommendations for HS treatment across eight clinically distinct populations, including pregnancy, breastfeeding, pediatrics, malignancy, TB infection, hepatitis B or C, and HIV. Strong recommendations reflect high-quality evidence with a clear benefit-risk balance, while conditional recommendations indicate areas where evidence is limited or clinical judgment is required. The guidance emphasizes safety, individualized treatment, and coordination with relevant specialists (e.g., obstetricians, oncologists, hepatologists, and infectious disease experts) when systemic therapies, immunomodulators, or biologics are indicated.
| Patient Population; Therapy | Recommendation Strength | Key Considerations |
|---|---|---|
| Pregnancy/planning pregnancy | ||
| Zinc supplementation | Strong | Evidence supports safety and benefit. |
| Avoid oral doxycycline | Strong | Risk of congenital anomalies |
| Avoid oral erythromycin | Strong | Risk of hepatotoxicity and other adverse outcomes |
| Metformin | Strong | Safe in pregnancy for HS management |
| Continuation of biologics | Strong | Maintaining disease control |
| Adalimumab for biologics | Strong | Preferred biologic when treatment needed |
| Limit doxycycline in breastfeeding | Strong | Reducing exposure to infant |
| Breastfeeding | ||
| Oral rifampin (systemic antibiotics) | Conditional | Using only if systemic therapy required |
| Antiandrogens: Metformin or oral contraceptives | Conditional | Considering risk/benefit for lactation |
| Prednisone < 20 mg/day (systemic immunomodulators/biologics) | Conditional | Only for acute, extensive flares |
| Pediatrics | ||
| Antiseptic washes with topical therapy | Conditional | Reducing bacterial resistance |
| Adolescent females: Spironolactone or combined oral contraceptives | Conditional | Considering developmental stage and safety |
| Biologics: Adalimumab ≥ 12 years | Strong | FDA-approved age group, effective in HS |
| Malignancy | ||
| Oral doxycycline (systemic antibiotics) | Strong | Safe for HS management post-cancer |
| Metformin (antiandrogens) | Strong | Preferred antiandrogen therapy |
| Systemic immunomodulators/biologics | Strong | Coordinating with oncologist, considering HS activity, prior cancer type/stage, prognosis, and immunosuppressive risk |
| TB infection | ||
| Rifampin 4-month course (latent TB) | Strong | Standard latent TB therapy |
| Antiandrogens: Metformin | Strong | Safe in high-risk TB patients |
| Glucocorticoids (> 15 mg prednisone eq.) | Strong | Annual latent TB screening recommended |
| Hepatitis B or C | ||
| Ciprofloxacin (systemic antibiotics, especially with cirrhosis) | Conditional | Adjustment for liver function |
| Antiandrogens (non-cirrhotic) | Strong | Using similar approach as general HS population |
| Systemic immunomodulators/biologics | Strong | Screening for hepatitis B/C prior to initiation |
| HIV | ||
| Oral doxycycline (systemic antibiotics) | Conditional | Safe choice in HIV patients |
| Antiandrogens | Conditional | Considering non-metformin antiandrogens; following general HS protocols |
| Systemic immunomodulators/biologics | Strong | HIV screening required prior to therapy |
Abbreviations: HS, hidradenitis suppurativa; TB, tuberculosis.
To sum up, the guidelines emphasize three key take-home messages:
1. Biologics can be safely administered for the treatment of HS during pregnancy and breastfeeding, ideally under close coordination with obstetric and pediatric specialists.
2. Biologic therapy is safe and effective for pediatric and adolescent patients with HS.
3. Patients with HS who have concurrent chronic infections can undergo HS treatment safely, provided there is careful collaboration with infectious disease specialists and other relevant clinicians.