1. Background
The completion of a full marathon has been associated with a decrease in physical function (1-3). Notably, an elevated risk of transient acute kidney injury (AKI) has been documented (1, 2). In our previous study, we reported an increase in renal injury markers within the context of full marathons (1). In response to AKI, apoptotic proximal tubular epithelial cells within the renal architecture exhibit robust expression of a transmembrane protein known as kidney injury molecule-1 (KIM-1) towards the luminal aspect (4-6). This prompts the apoptosis inhibitor of macrophages (AIM) to circulate in the bloodstream and bind with KIM-1. This cascade induces macrophages to undergo phagocytosis and eliminate deceased cellular constituents (7). This intricate orchestration results in a decrease in blood AIM levels and a corresponding increase in urinary AIM levels. Additionally, the KIM-1 levels in the urinary matrix exhibit a notable increase during AKI. Consequently, urinary AIM and KIM-1 have the potential to serve as non-invasive biomarkers for the assessment of renal damage. However, these dynamics remain unclear in the context of marathon-like physical activity.
Although our previous research has demonstrated that certain biomarkers in either plasma or urine fluctuate in response to marathon-induced stress, such findings have typically been limited to individual molecules within a single biological compartment. It is rare to identify a biomarker that can be evaluated simultaneously in both blood and urine within the same molecular entity. From this perspective, AIM is a particularly valuable candidate, as it offers a dual-compartment assessment within a single molecule — an uncommon and informative feature for evaluating exercise-induced AKI.
2. Objectives
The objective of our investigation was to measure circulating and urinary AIM and urinary KIM-1 levels, complementing our previous study (1), to examine their potential utility as biomarkers.
3. Methods
3.1. Participants
We examined 23 young men (23 ± 1 years of age, 172 ± 5 cm in height, and 63 ± 4 kg in body weight) participating in the 38th Tsukuba Marathon (1). All individuals in this group followed a consistent routine of systematic physical training, exhibited no inclination for tobacco consumption, maintained a lean physique, and abstained from using pharmacological agents.
3.2. Experimental Protocol
Participants were instructed to refrain from consuming any food or alcohol intake for ≤ 12 hours before baseline measurements. Pre-marathon (before) blood and spot urine samples were collected in the morning following sufficient rest. Post-marathon (after) blood and urine sampling occurred immediately (within 30 minutes) after completing the race. These samples were stored at -80℃ until chemical analyses.
3.3. Plasma and Urinary Apoptosis Inhibitor of Macrophages and Urinary Apoptosis Inhibitor of Macrophages Levels
Plasma and urinary AIM levels were assessed using the Human AIM/CD5L Assay Kit (ImmunoBiological Laboratories Co., Ltd., Gunma, Japan). Urinary KIM-1 levels were assessed using a KIM-1 (human) ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA). All assays were performed following the manufacturer’s instructions.
3.4. Statistical Analysis
Data are presented as mean ± standard deviation (SD). The t-test was employed for all measurements, and for significant F-values, comparisons were made using Tukey's post-hoc test. Correlations were used to examine the bivariate associations between the relative changes in plasma and urinary parameters before and after the full marathon. GraphPad Prism 7 software (GraphPad, Inc., La Jolla, CA, USA) was used for all statistical calculations, and the significance level was set at P < 0.05 in all cases.
4. Results
On the marathon day, the weather was sunny, with an average temperature of 12.7℃ and an average humidity level of 60.6%. All runners completed 42.195 km, with an average marathon finishing time of 247 ± 56 minutes. Serum creatinine levels significantly increased from 0.86 ± 0.11 mg/dL to 1.09 ± 0.27 mg/dL (P = 0.0001), and urinary creatinine levels also showed a significant increase from 208.0 ± 62.3 mg/dL to 283.5 ± 162.8 mg/dL (P = 0.041) following the full marathon, based on previously published data (1). Plasma AIM levels (5.3 ± 2.1 vs. 4.5 ± 1.7 pg/mL) significantly decreased, and urinary AIM levels (0.21 ± 0.25 vs. 0.99 ± 1.10 pg/mL/g creatinine) and urinary KIM-1 levels (99.0 ± 40.9 vs. 136.9 ± 74.1 pg/mL/g creatinine) significantly increased immediately following the marathon run (Figure 1).
Table 1 presents simple correlations among the changes in plasma and urinary AIM and urinary KIM-1 levels before and immediately after the full marathon. A positive correlation was observed between the changes in urinary AIM levels and corresponding changes in urinary KIM-1 levels (R = 0.560, P = 0.005).
| Variables | Plasma AIM | Urinary AIM | Urinary KIM-1 |
|---|---|---|---|
| Plasma AIM | - | -0.081 (P = 0.713) | 0.133 (P = 0.546) |
| Urinary AIM | - | - | 0.560 (P = 0.005) |
| Urinary KIM-1 | - | - | - |
Abbreviations: AIM, apoptosis inhibitor of macrophage; KIM-1, kidney injury molecule-1.
5. Discussion
This study revealed an intriguing phenomenon, wherein urinary AIM levels exhibited a surge concurrent with a decrease in plasma AIM levels, although not significantly correlated. Remarkably, an increase in urinary AIM levels was accompanied by a notable rise in urinary KIM-1 levels. Our previous results revealed that urinary albumin and liver-type fatty acid-binding protein (L-FABP) levels are elevated, and the estimated glomerular filtration rate derived from serum creatinine decreases following a full marathon (1). Additionally, this observation is not exclusive to our study but aligns with a compendium of previous inquiries that have confirmed changes in renal markers due to long-distance running, such as a full marathon (1, 2). These results converge and irrefutably support transient AKI precipitated by the rigor of a full marathon. These biomarkers — albumin, L-FABP, and creatinine — are widely recognized as indicators of AKI. Notably, AIM is unique in that it exists in both the blood and urine, allowing for dual-compartment assessment. This characteristic enhances its potential as a reliable and comprehensive biomarker for exercise-induced AKI.
Urinary KIM-1 levels, which indicate an increase during apoptotic renal injury, have been observed to rise in the urinary system (4). Importantly, this study not only demonstrated an increase following a full marathon but also revealed a positive correlation between urinary AIM and KIM-1 levels. These elevations in AIM and KIM-1 levels in the urine suggest a collaborative role in mitigating proximal tubular injury caused by exercise-induced AKI. Moreover, a prolonged increase in urinary KIM-1 levels is associated with chronic inflammation, which can lead to renal fibrosis (8). Consequently, whether the prompt return of urinary KIM-1 levels to baseline after exercise-induced renal stress should be investigated in future studies. Since AIM acts as a ligand that binds to KIM-1 during proximal tubular injury, their combined presence in urine may represent a biologically meaningful signature of renal epithelial stress. This highlights the potential of urinary measurements to reflect localized renal processes more accurately than plasma levels.
The AIM normally remains sequestered within the bloodstream as it forms a complex with Immunoglobulin M pentamers (9). However, during conditions such as AKI, it undergoes liberation from IgM, facilitating ligand-receptor interactions with KIM-1 in the kidney (7). This study suggests that free AIM plays a crucial role in the kidney by partnering with KIM-1 to clear cellular debris during vigorous running, as indicated by the positive correlation between urinary AIM and KIM-1 levels. However, no significant inverse correlation was observed between plasma and urinary AIM levels. This discrepancy may be partly attributed to individual variability in the rate and extent of AIM dissociation from IgM. Exercise-induced physiological changes — such as altered pH, oxidative stress, or transient changes in renal perfusion — may influence disulfide bond stability and the conformational dynamics of the IgM-AIM complex, thereby affecting AIM release kinetics. Additionally, the accumulation of AIM within the bladder before excretion may confound temporal associations between blood and urine levels. Therefore, factors such as individual hydration status during the race and frequency of urination — which were not controlled or recorded in this study — may influence urinary biomarker concentrations and should be carefully considered in future research to enhance the accuracy and interpretability of the findings.
This study has two main limitations. The first is that only male amateur runners were included as participants. This exclusion of female runners was due to the potential confounding effect of the menstrual cycle, which may influence biomarkers such as AIM and KIM-1. Consequently, the findings are limited to male participants, and the generalizability of the results to female athletes is uncertain. Future studies should include female participants and account for menstrual cycle phases to better understand gender-specific responses to marathon-induced renal stress.
The second limitation is that the measurements were not performed under strictly controlled laboratory conditions. By implementing a controlled environment and collecting serial data at multiple time points (e.g., after 24 and 48 hours post-exercise), future studies could enhance the reliability and temporal characterization of biomarker responses. Furthermore, including a broader panel of biomarkers — such as albumin and L-FABP, in addition to AIM and KIM-1 — may provide a more comprehensive understanding of exercise-induced AKI.
5.1. Conclusion
In conclusion, this study revealed a significant increase in urinary AIM levels in amateur runners after a full marathon. The increase was also evident as a positive correlation with urinary KIM-1 levels, interacting with the proximal tubule and thus serving as a marker of renal damage. Such approaches could strengthen their applicability in clinical and sports medicine settings for monitoring exercise-induced kidney stress.
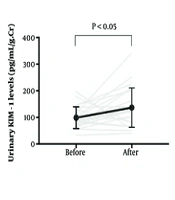
![Response of plasma apoptosis inhibitor of macrophages (AIM), urinary AIM, and urinary kidney injury molecule-1 (KIM-1) levels to the marathon run [black circles represent means ± standard deviation (SD); gray lines represent individual changes]. Response of plasma apoptosis inhibitor of macrophages (AIM), urinary AIM, and urinary kidney injury molecule-1 (KIM-1) levels to the marathon run [black circles represent means ± standard deviation (SD); gray lines represent individual changes].](https://services.brieflands.com/cdn/serve/3170d/8d369f05f54e129e0616c7a99c480a955c944a80/asjsm-16-1-161117-i001-preview.webp)