1. Background
Hepatitis B virus (HBV) is a DNA virus from the Hepadnaviridae family and remains a significant global public health concern, affecting nearly 300 million people worldwide (1). Approximately one-third of the global population has been exposed to HBV, which is a leading cause of severe liver diseases, including cirrhosis and hepatocellular carcinoma (1). Chronic hepatitis B (CHB) is responsible for approximately 800,000 deaths annually (2). The HBV can be transmitted through parenteral, sexual, horizontal, and vertical routes, with vertical transmission — defined as mother-to-child transmission during pregnancy, delivery, or postpartum — playing a critical role in the spread of the virus (3). In hepatitis B e antigen (HBeAg) positive mothers, the risk of transmitting HBV to their infants can reach up to 90% (4). Furthermore, 85 - 95% of infants infected perinatally are at risk of developing chronic HBV infection. However, vertical transmission can be significantly reduced through appropriate interventions, including regular follow-up of pregnant women with CHB, initiation of antiviral therapy during pregnancy when indicated, and administration of both hepatitis B immunoglobulin (HBIG) and the hepatitis B vaccine to newborns (5). Despite these measures, the risk of transmission cannot be entirely eliminated (6).
Current guidelines (7, 8) recommend that hepatitis B surface antigen (HBsAg)-positive pregnant women undergo HBV DNA and ALT level monitoring during each trimester. If HBV DNA levels exceed 200,000 IU/mL, antiviral therapy should be initiated between 24 and 28 weeks of pregnancy (9). Tenofovir-based therapies, such as tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF), are the preferred antiviral agents during pregnancy (10). In Turkey, the introduction of the hepatitis B vaccine in 1998 has led to a decline in CHB seroprevalence (11). However, the country remains classified as having intermediate CHB prevalence, with an HBsAg seroprevalence of approximately 4% (12). Regional variations exist, with the Southeastern Anatolia region reporting a higher seroprevalence of 7.3% (13). The durability of vaccine-induced protection remains an important concern. A large Turkish cross-sectional study assessing children nine years after primary immunization found that nearly half had anti-HBs titers below protective levels, despite receiving the full three-dose schedule at birth (14).
2. Objectives
This study aims to evaluate the extent to which pregnant women diagnosed with CHB who gave birth in our hospital between 2017 and 2022, and their newborns, were followed up in accordance with current guidelines. By assessing real-life clinical practices, this study seeks to identify gaps in care and provide insights for improving adherence to HBV management protocols.
3. Methods
3.1. Study Design and Data Collection
This retrospective study was conducted at the Sakarya University Training and Research Hospital in Turkey. Our institution is a tertiary care hospital with around 900 beds. The study period spanned from January 1, 2017, to December 31, 2022. Patient data were collected and analyzed using the hospital's data processing system. The study included patients who were followed up with a diagnosis of CHB in the infectious diseases outpatient clinic and who gave birth in the obstetrics and gynecology clinic of our hospital during the study period. All tests were performed in the same hospital laboratory using standard commercial assays. A uniform cutoff value of anti-HBs ≥ 10 mIU/mL was used to define protective immunity, in line with international recommendations.
The inclusion criteria were as follows:
- Pregnant women diagnosed with CHB who gave birth in our hospital between January 1, 2017, and December 31, 2022.
- Availability of complete medical records for both mothers and their newborns.
Patients were excluded if they experienced miscarriage or stillbirth, gave birth at another medical center, or if their medical records were incomplete or missing essential data.
We identified newborns of the included patients through the hospital data processing system. Babies were meticulously checked to determine whether they had received HBIG and the hepatitis B vaccine at birth. Additionally, any available test results for HBsAg, anti-HBs, and anti-HBc IgG in the babies at any time during the study period were recorded and analyzed. The primary outcomes measured included:
- The proportion of newborns receiving HBIG and the hepatitis B vaccine at birth.
- The proportion of newborns tested for HBsAg, anti-HBs, and anti-HBc IgG, and the seroprevalence of these tests in the newborns.
Since the HBV DNA level and HBeAg status of mothers have a significant effect on vertical transmission, these parameters were also examined in the study. We analyzed data of HBsAg-positive pregnant women regarding HBeAg status, HBV DNA levels during pregnancy, and antiviral treatment during pregnancy.
3.2. Ethical Considerations
Ethical approval for the study was obtained from the Sakarya University Faculty of Medicine Non-Interventional Ethics Committee on October 31, 2023, with approval number 318. Due to the retrospective nature of the study, informed consent was not obtained from the patients. Patient confidentiality and data privacy were maintained throughout the study. The study was conducted in accordance with the principles of the Declaration of Helsinki.
3.3. Statistical Analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. The rates of HBIG and hepatitis B vaccine administration, as well as the serological test results for the newborns, were calculated and reported. Categorical variables were analyzed using the chi-square (χ2) test, while continuous variables were evaluated with the Mann-Whitney U test. A P-value of < 0.05 was considered statistically significant.
4. Results
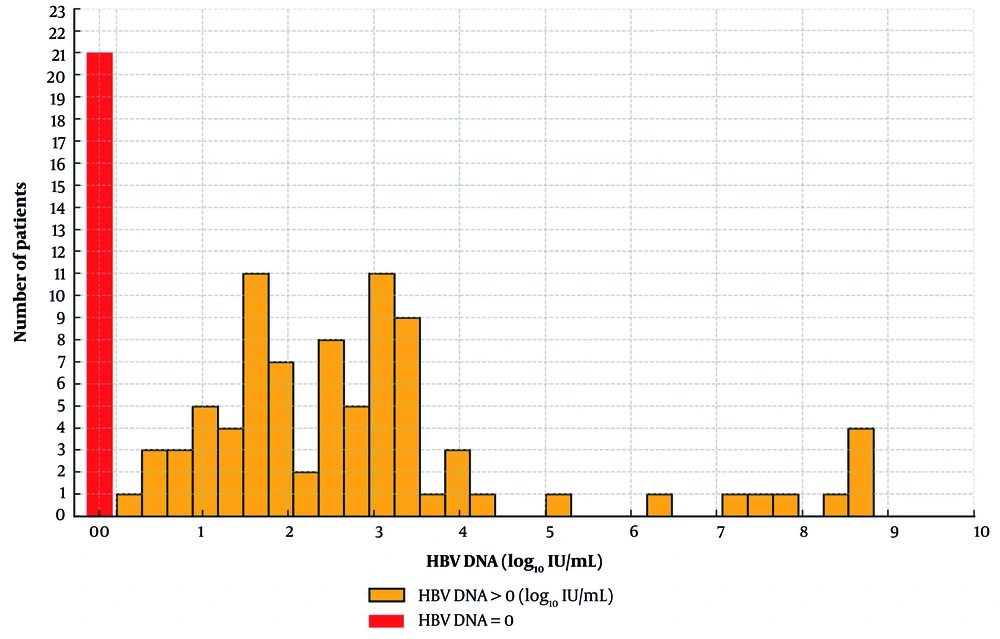
During the study period, data from 177 women were analyzed. However, 40 women were excluded due to miscarriage, stillbirth, or delivery at another center. Among the 137 women included in the study, 113 had a single delivery, 18 had two deliveries, and 6 had three deliveries, resulting in a total of 167 babies born within the study period. A total of 111 women were tested for HBeAg during 140 pregnancies. In 12 pregnancies, HBeAg was positive (8.5%). The HBV DNA results were available in 105 pregnancies (62.8%). The median HBV DNA level was 92 IU/mL, with an interquartile range (IQR) of 1,666 IU/mL. The distribution of HBV DNA levels is shown in Figure 1.
Eighteen different mothers had antiviral use in 19 pregnancies (11.3%), and all received TDF. In seven pregnant women, TDF treatment was started during pregnancy. Six had high HBV DNA levels, and one had a history of HBsAg-positive children. There was no record of antiviral use in three pregnant women with HBV DNA levels above 200,000 IU/mL. There was no significant association between the availability of HBeAg and HBV DNA results during pregnancy and the presence of anti-HBs titer results in babies (P = 0.689 and P = 0.541, respectively). A significant correlation was observed between receiving antiviral therapy and the presence of anti-HBs titer testing, which was more commonly performed among patients who received treatment (P < 0.001). There was no significant association between anti-HBs titer positivity and HBeAg status, HBV DNA level, or antiviral treatment status (P = 1).
All 167 babies born between 2017 and 2022 received the first dose of the hepatitis B vaccine at birth. Additionally, 163 babies (97.6%) had records indicating they received HBIG at birth. Eight babies were excluded from the hepatitis B serology evaluation due to a lack of postnatal records in our hospital. Of the remaining 159 infants:
- Twenty were tested for anti-HBs.
- Nineteen were tested for HBsAg.
- One was tested for anti-HBc IgG.
Among the 20 infants tested for anti-HBs:
- Fifteen tested positive for anti-HBs.
- Five tested negative for anti-HBs (< 10 mIU/mL).
- One infant initially tested negative for anti-HBs but later tested positive.
All infants tested for HBsAg and anti-HBc IgG had negative results. Anti-HBs was tested in 20 (12.5%) of the 159 infants. Notably, 3 of these 20 infants were tested before 9 months of age, and all 3 were positive. The remaining 17 (10.6%) infants were tested at a median age of 31 months, with 12 (70.5%) testing positive for anti-HBs. Demographic and numerical data of the pregnant women and their infants examined in the study are shown in Table 1.
| Feature | No. (%) |
|---|---|
| Number of female cases | 137 |
| Total number of babies born | 167 |
| Number of babies vaccinated at birth | 167 (100) |
| Number of babies received HBIG at birth | 163 (97.6) |
| Male gender of babies | 89 (53.2) |
| Total number of babies evaluated for HBV serology | 159 |
| Number of babies tested for anti-HBS | 20 (12.5) |
| Number of infants tested for anti-HBS in the appropriate period | 17 (10.6) |
| Anti-HBS positivity rate (n = 17) | 12 (70.5) |
| Number of infants requested HBsAg | 19 (11.9) |
| HBsAg positivity rate | 0 (0) |
Abbreviations: HBIG, hepatitis B Immunoglobulin; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen.
5. Discussion
This study provides a real-world snapshot of the follow-up and serological monitoring practices for infants born to HBsAg-positive mothers in our center. Our study offers valuable insights into the follow-up practices and serological testing of infants born to mothers with CHB. Among the 167 infants born to 137 women during the study period, data completeness was high, with records available for 159 infants (95.2%). However, only 20 infants (12.5%) underwent anti-HBs testing, and 3 of these were tested before the recommended age of 9 months, contrary to current guidelines (15). Various studies worldwide have shown that postnatal serologic follow-up is not performed at the desired level in infants born to HBsAg-positive mothers. For instance, even after a new follow-up program in Sweden, the postnatal serological follow-up rate reached 92% (16), while this rate is below 50% in many countries (17). A recent study from the United Kingdom showed that timely birth-dose vaccination, HBIG for high-risk infants, and maternal tenofovir therapy resulted in a 0% vertical transmission rate, though challenges remain with loss to follow-up among high-risk infants and the need for optimized viral load monitoring and appropriately timed serological testing (18). According to the World Health Organization's 2024 report, it is critical to expand postnatal serological follow-up to achieve HBV elimination targets, but this rate is below 20% in many countries (7). In Turkey, there are very few studies on postnatal serologic follow-up and seroconversion rates, and the available data show that the practice is far behind the guideline recommendations. Among the infants tested, 70.5% developed protective anti-HBs antibodies, which is lower than the rates reported in other countries. For instance, a study conducted in Chile, a low-prevalence country, reported a 96% anti-HBs positivity rate at 9 - 12 months (17). Similarly, in Sweden, the implementation of a new vaccination program for HBV-infected mothers and their infants increased follow-up rates from 63% to 92%, with a 99% anti-HBs positivity rate (16). Long-term follow-up data from the same program revealed that 82% of children aged 8 - 12 years maintained protective anti-HBs levels (19). A cross-sectional study of Turkish children nine years after infant hepatitis B vaccination found that nearly half had anti-HBs titers below protective levels (14). These findings suggest that while the vaccination and immunoglobulin administration protocols in our study were largely followed, the lower rate of anti-HBs positivity may reflect gaps in follow-up care, timing of testing, or other factors such as vaccine response variability.
The causes of insufficient follow-up in Turkey seem to be complex and multifactorial. At the systemic level, there is a lack of a standardized national protocol or a digital reminder system that ensures infants born to HBsAg-positive mothers are consistently tested. Factors related to physicians also contribute, as the responsibility for follow-up is frequently unclear among obstetricians, pediatricians, and specialists in infectious diseases. Additionally, family-related factors, such as limited awareness and socioeconomic challenges, may play a role in the low rates of testing. Addressing these issues necessitates a coordinated approach that includes electronic registries, automated reminders integrated into national health systems, and comprehensive educational programs for both healthcare providers and families (20).
The results of this study have widespread implications for public health. Without confirming seroconversion, unprotected infants are left at risk for chronic HBV infection and are denied the opportunity to reach national hepatitis B prevention goals. These gaps in care are critical for individual childhood lives and in achieving WHO targets for HBV elimination by 2030 (7). Studies have shown that revaccination can be effective in achieving seroconversion in infants who initially fail to develop protective antibody levels (21). Another finding in this study is that three pregnant women with high HBV-DNA levels appear not to have received antiviral treatment. They may have had late diagnoses, incomplete records, treatment contraindications, or refusal. In this context, organizing training programs for healthcare professionals can help raise awareness about the prevention and management of HBV infection. Additionally, families need to be informed to play an active role in this process. Specifically, educating HBsAg-positive mothers about the importance of postnatal follow-up for their infants can ensure the complete implementation of vaccination and immunoprophylaxis practices. Such awareness-raising initiatives can make a significant contribution to preventing vertical transmission of HBV at both individual and societal levels (22). A recent European survey revealed considerable disparities and irregularities in hospital protocols aimed at preventing vertical transmission of hepatitis B (23). These findings highlight the urgent need for a harmonized, multidisciplinary approach to care. This study found that anti-HBs testing in infants was more commonly performed when the mother was receiving antiviral therapy during pregnancy. This may be explained by the fact that these individuals are followed more closely and attend the clinic more frequently for prescription renewals, providing more opportunities for counseling and testing of the child. Another controversial issue in preventing vertical transmission of hepatitis B is the mode of delivery. Current literature indicates that the mode of delivery is not significant in transmission when immunoprophylaxis is correctly administered (7). The mode of delivery was not examined in this study.
A significant limitation of our study is its retrospective design, which relies on existing medical records. This can lead to bias due to missing data or incomplete follow-up data. For example, some infants may have been tested at other healthcare facilities, leading to an underestimation of follow-up rates. Additionally, the study was conducted at a single center, which limits the generalizability of our findings. Future prospective multicenter studies are warranted to validate these findings and assess the consistency of follow-up practices across different regions and healthcare systems. Also, the timing of anti-HBs testing in our study was inconsistent, with some infants tested before the recommended age of 9 months. Future research should focus on the long-term outcomes of infants born to HBsAg-positive mothers, including the durability of protective anti-HBs levels and the effectiveness of revaccination strategies. Exploring the barriers to guideline adherence could inform targeted interventions to improve follow-up practices.
This study emphasizes the urgent need to adhere to guidelines for serological monitoring and vaccination for infants born to HBsAg-positive mothers in order to prevent vertical HBV transmission. Major gaps in follow-up care and guideline adherence were highlighted concerning anti-HBs testing and long-term monitoring, especially with respect to timing. Addressing these issues through the systematic implementation of follow-up protocols, training of healthcare providers, and educating families will help raise vaccination coverage and minimize risks of transmissibility. Large multicenter studies should be encouraged in the near future to bring these issues into focus more clearly and optimize HBV preventive strategies for Turkey.