1. Background
Iron deficiency anemia (IDA) in children has become a global health concern, which may result from suboptimal dietary patterns but can also indicate the presence of more serious underlying diseases. If left untreated, IDA can adversely affect psychomotor, cognitive, and behavioral development, since iron is an essential mineral required for brain development and function (1-3). In Indonesia, anemia remains one of the most prevalent nutritional problems, particularly among school-aged children and adolescents, mainly due to deficiencies in both macronutrients and micronutrients, especially iron (4). The IDA among adolescent girls has both immediate and long-term health consequences, including reduced concentration and memory, lower school attendance, impaired physical growth, delayed menarche, decreased immunity, and reduced physical endurance (5). If this condition persists into pregnancy, it increases the risk of low birth weight (LBW), miscarriage, hemorrhage during pregnancy and childbirth, impaired intrauterine growth and development, stunting, lower intelligence, and higher infant mortality (6).
Data from the World Health Organization (WHO) indicate that Africa and Southeast Asia are the two regions with the highest burden of anemia worldwide, with approximately 106 million women and 103 million children affected in Africa, and 244 million women and 83 million children in Southeast Asia. Globally, the prevalence of anemia among women of reproductive age (15 - 49 years) in 2019 reached 29.9%, with a nearly similar rate of 29.6% among non-pregnant women in the same age group, which includes adolescent girls (7). In Indonesia, the 2018 Basic Health Research (Riskesdas) report stated that the prevalence of anemia among adolescent girls aged 15 - 24 years was 27.2%, compared to 20.3% among adolescent boys (8). This indicates that adolescent girls are more vulnerable to anemia and, therefore, require special attention in health promotion and prevention efforts.
Anemia results primarily from iron deficiency (7). Contributing factors include insufficient dietary iron intake, low bioavailability, increased physiological needs, blood loss, unbalanced dietary patterns, infectious diseases, low nutritional knowledge, and poor socioeconomic conditions. Iron absorption is further influenced by dietary inhibitors and enhancers. Inhibitors include caffeine, tannins, oxalates, and phytates, which are found in soy products, tea, coffee, and whole grains. Enhancers include vitamin C (present in citrus fruits and papaya) and animal proteins such as beef, chicken, and fish.
To address anemia in adolescents, the government, through community health centers (Puskesmas), has introduced weekly iron and folic acid supplementation programs (four tablets per month). However, the main challenge remains adherence, which may contribute to the persistently high prevalence of anemia among adolescent girls. Compliance with supplementation and adequate nutritional knowledge are critical for raising hemoglobin (Hb) levels and preventing anemia (9).
The Indonesian government has implemented various programs, including iron supplementation targeting adolescent girls and pregnant women, to combat anemia. Nonetheless, challenges such as low adherence and limited health education persist, requiring further interventions to improve public health outcomes and reduce the prevalence of anemia (10). The WHO also recommends several interventions to prevent and manage anemia in vulnerable groups, including infants, young children, menstruating adolescent girls and women, and pregnant and postpartum women. Daily iron supplementation reduces the risk of anemia in infants, children, and pregnant women, while intermittent supplementation is effective for menstruating girls and women (11). Current supplementation programs will not effectively increase iron status unless they are accompanied by good compliance and attention to dietary patterns that influence iron absorption. To date, no research in Bandung has specifically analyzed dietary consumption patterns and adherence to iron supplementation among adolescent girls.
2. Objectives
This study aimed to examine the association between dietary patterns — specifically, the intake of enhancers and inhibitors of iron absorption — and anemia status in adolescent girls.
3. Materials and Methods
This cross-sectional, descriptive-analytic study was conducted from January to March 2025 at a public junior high school in Bandung, West Java, Indonesia, involving 63 adolescent girls aged 13 - 15 years who were selected through simple random sampling. The sample size was determined using the Lemeshow formula, based on an estimated anemia prevalence of 27% among Indonesian adolescent girls and a 10% margin of error. Participants were eligible if they were healthy, provided parental consent, and were not taking iron supplements; those with chronic illness or incomplete data were excluded.
The Hb levels were measured using a HemoCue® Hb 201+ photometer (HemoCue AB, Angelholm, Sweden) and categorized according to WHO criteria (< 12 g/dL = anemia). Dietary intake data were collected using a validated semi-quantitative Food Frequency Questionnaire (FFQ) assessing the frequency of consumption of enhancers (heme iron, non-heme iron, vitamin C, fructose) and inhibitors (tannins, polyphenols, calcium, phytates) of iron absorption, with results expressed in milligrams per day. Data normality was assessed using the Kolmogorov-Smirnov test, and associations between nutrient intake and anemia status were analyzed using the chi-square test with a significance level of P < 0.05.
To minimize bias, enumerators received standardized training, instruments were pretested (Cronbach’s α = 0.82), and Hb measurements were supervised by trained technicians with daily calibration of the device. Ethical approval was obtained from the Research Ethics Committee of Universitas Pendidikan Indonesia (No. UPI-KEPK/2025/042). This study was funded by a Research Grant from Universitas Pendidikan Indonesia, Faculty of Sport and Health Education (grant No. 2025-UPI).
4. Results
The frequency distribution analysis revealed that the majority of respondents were 14 years old (57.1%), followed by those aged 13 years (31.7%) and 15 years (11.1%, Table 1). This finding indicates that most participants were in early adolescence, a critical developmental stage that requires optimal nutritional intake, particularly of iron.
| Variables | No. (%) |
|---|---|
| Age (y) | |
| 13 | 20 (31.7) |
| 14 | 36 (57.1) |
| 15 | 7 (11.1) |
| Hb (g/dL) | |
| Severe anemia (< 8) | 3 (4.8) |
| Moderate anemia (8.0 - 10.9) | 9 (14.3) |
| Mild anemia (11.0 - 11.9) | 14 (22.2) |
| Normal (≥ 12) | 37 (58.7) |
| Menstrual regularity | |
| Regular | 51 (81.0) |
| Irregular | 12 (19.0) |
| Duration of menstruation (d) | |
| < 7 | 35 (55.6) |
| > 7 | 28 (44.4) |
| Consumption of iron tablets | |
| 1 week ago | 6 (9.5) |
| 2 weeks ago | 1 (1.6) |
| 1 month ago | 4 (6.3) |
| 2 months ago | 6 (9.5) |
| Never | 46 (73) |
Abbreviation: Hb, hemoglobin.
4.1. Enhancer
A significant association was observed between the consumption of iron absorption enhancers — heme iron, non-heme iron, vitamin C, and fructose — and anemia status (P = 0.00 for all variables, Table 2). This finding supports the theory that adequate intake of iron absorption enhancers has a strong influence on Hb status, especially in vulnerable groups such as adolescent girls.
| Variables | Hb Levels (g/dL) | Total | P-Value | |
|---|---|---|---|---|
| Anemia | Non-anemia | |||
| Heme iron intake (mg/d) | 0.00 | |||
| Occasionally | 10 (40) | 15 (60) | 25 (100) | |
| Moderately frequent | 19 (51.4) | 18 (48.6) | 37 (100) | |
| Frequent | 1 (100) | 0 (0.0) | 1 (100) | |
| Non-heme iron intake (mg/d) | 0.00 | |||
| Occasionally | 11 (50) | 11 (50) | 22 (100) | |
| Moderately frequent | 15 (44.1) | 19 (55.9) | 34 (100) | |
| Frequent | 4 (57.1) | 3 (42.9) | 7 (100) | |
| Vitamin C intake (mg/d) | 0.00 | |||
| Rare | 1 (100) | 0 (0.0) | 1 (100) | |
| Occasional | 12 (36.4) | 21 (63.6) | 33 (100) | |
| Moderately frequent | 17 (58.6) | 12 (41.4) | 29 (100) | |
| Fructose intake (mg/d) | 0.00 | |||
| Rare | 1 (100) | 0 (0.0) | 1 (100) | |
| Occasional | 13 (43.3) | 17 (56.7) | 30 (100) | |
| Moderately frequent | 14 (48.3) | 15 (51.7) | 29 (100) | |
| Frequent | 2 (66.7) | 1 (33.3) | 3 (100) | |
Abbreviation: Hb, hemoglobin.
a Values are expressed as No. (%).
4.2. Inhibit
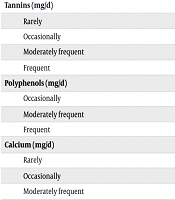
Based on the frequency distribution analysis, the intake of inhibitors such as tannins, polyphenols, calcium, and phytates showed no statistically significant association with anemia status among adolescent girls (all P > 0.05, Table 3).
| Variables | Hb Levels (g/dL) | Total | P-Value | |
|---|---|---|---|---|
| Anemia | Non-anemia | |||
| Tannins (mg/d) | 0.196 | |||
| Rarely | 3 (60) | 2 (40) | 5 (100) | |
| Occasionally | 11 (34.4) | 21 (65.6) | 32 (100) | |
| Moderately frequent | 15 (62.5) | 9 (37.5) | 24 (100) | |
| Frequent | 1 (50) | 1 (50) | 2 (100) | |
| Polyphenols (mg/d) | 0.913 | |||
| Occasionally | 13 (50) | 13 (50) | 26 (100) | |
| Moderately frequent | 15 (46.9) | 17 (53.1) | 32 (100) | |
| Frequent | 2 (40) | 3 (60) | 5 (100) | |
| Calcium (mg/d) | 0.574 | |||
| Rarely | 1 (100) | 0 (0.0) | 1 (100) | |
| Occasionally | 4 (36.4) | 7 (63.6) | 11 (100) | |
| Moderately frequent | 19 (51.4) | 18 (48.6) | 37 (100) | |
| Frequent | 6 (42.9) | 8 (57.1) | 14 (100) | |
| Phytates (mg/d) | 0.321 | |||
| Rarely | 0 (0.0) | 1 (100) | 1 (100) | |
| Occasionally | 13 (56.5) | 10 (43.5) | 23 (100) | |
| Moderately frequent | 17 (45.9) | 20 (54.1) | 37 (100) | |
| Frequent | 0 (0.0) | 2 (100) | 2 (100) | |
Abbreviation: Hb, hemoglobin.
a Values are expressed as No. (%).
5. Discussion
This finding indicates that most participants were in early adolescence, a critical developmental stage requiring optimal nutritional intake, particularly iron. According to the WHO, adolescent girls are highly vulnerable to anemia due to the combined effects of rapid growth and the onset of menstruation (7). The present study highlights the multifactorial nature of IDA among adolescent girls, emphasizing the significant roles of physiological, nutritional, and behavioral factors. Consistent with existing literature, the high prevalence of anemia despite ongoing iron-folic acid (IFA) supplementation programs suggests that additional determinants — including dietary patterns and adherence to supplementation — require further attention (7).
Rahmati et al. demonstrated that Hb concentration and obstetric history, particularly previous abortions, are key indicators correlated with iron deficiency in pregnant women without anemia during their first trimester (12). These findings align with the vulnerability of adolescent populations, who experience rapid growth and menstrual iron loss, thereby increasing susceptibility to depletion of iron stores. The observation in this study that Hb levels are substantially related to ferritin status supports our findings, indicating that adherence to supplementation and iron bioavailability from the diet are crucial modulators of anemia risk.
Complementing these clinical insights, Zhou et al. utilized a Mendelian randomization approach and reported no causal relationship between Helicobacter pylori infection and IDA, suggesting that H. pylori may not be a direct contributor to IDA among general populations without significant gastrointestinal pathology (13). This assertion refines previous epidemiological evidence implicating H. pylori infection as a risk factor, emphasizing the need for cautious interpretation of non-genetic associative studies and encouraging further exploration of mechanisms affecting iron metabolism.
The synergistic interpretation of these findings, together with our data on the significant influence of dietary enhancers — heme iron, non-heme iron, vitamin C, and fructose — and inhibitors such as tannins and phytates, underscores the necessity for targeted nutritional interventions that optimize iron absorption. The low adherence rates to iron supplementation programs further highlight behavioral barriers that must be addressed through effective educational strategies.
Overall, this integrative review underscores the complexity of IDA etiology, advocating for a comprehensive approach that includes clinical monitoring of hematological indices, assessment of infection status where relevant, promotion of dietary behaviors enhancing iron bioavailability, and sustained efforts to improve compliance with supplementation among at-risk adolescent and maternal populations.
The Hb measurements in this study showed that 41.3% of respondents were anemic, including mild (22.2%), moderate (14.3%), and severe (4.8%) cases, while 58.7% had normal Hb levels (≥ 12 g/dL). Despite the implementation of the IFA supplementation program, the relatively high anemia prevalence highlights the need to evaluate additional determinants such as dietary patterns and adherence to supplementation (14). Paramita et al. emphasized that iron absorption is not only influenced by IFA intake but also by dietary inhibitors, such as tannins in tea and calcium in milk (15).
Menstrual patterns further contribute to iron status. Most respondents (81%) reported regular menstruation, while 19% experienced irregular cycles. The majority (55.6%) reported a menstrual duration of less than 7 days, whereas 44.4% reported menstruation lasting more than 7 days. Menstrual bleeding is a key source of iron loss; Badenhorst et al. estimated that women may lose approximately 1 mg of iron daily during menstruation, thereby increasing anemia risk if not balanced with adequate dietary intake (16).
Adherence to IFA supplementation was also notably poor. A substantial proportion of respondents (73%) reported never taking IFA tablets, while only a minority consumed them in the past 1 week (9.5%), 2 weeks (1.6%), 1 month (6.3%), or 2 months (9.5%). Low adherence significantly contributes to the high prevalence of anemia, consistent with findings by Mukharomah and Budiono (17), who highlighted that the effectiveness of supplementation programs depends on consistent intake, which is closely linked to knowledge, attitudes, and perceptions of side effects. Additionally, supplementation effectiveness is influenced by dosage, duration, and scheduling. Andersen et al. noted that optimal hematological response is observed in individuals with overt iron deficiency, while chronic inflammation can impair absorption and mobilization despite adequate iron stores (18).
Overall, the findings indicate that, in addition to physiological factors such as age and menstruation, both dietary patterns and adherence to IFA supplementation play central roles in iron absorption and anemia status among adolescent girls. Educational and behavioral interventions are therefore essential to improve compliance with supplementation and promote dietary practices that enhance iron absorption.
Heme iron, found in animal products such as red meat, liver, poultry, and fish, demonstrated the strongest association with anemia. Respondents who consumed heme iron “frequently” reported no anemia, whereas those who consumed it “occasionally” exhibited a higher prevalence of anemia (40%). This is consistent with the reported bioavailability of heme iron (15 - 35%), which is substantially higher than that of non-heme iron (2 - 20%) (19, 20).
Non-heme iron, present in plant-based foods such as leafy vegetables, legumes, and grains, also showed significant associations. Respondents who consumed non-heme iron “fairly often” had a lower prevalence of anemia (44.1%) compared to those consuming it “occasionally” (50%). These findings are in line with Labba et al., who demonstrated that regular non-heme iron intake improves Hb levels, particularly when combined with vitamin C. National surveys have similarly reported lower anemia risk among adolescent girls who regularly consume vegetables and legumes (21).
Vitamin C intake played a crucial role as a potent enhancer of non-heme iron absorption by reducing ferric (Fe3+) to ferrous (Fe2+), the more soluble and absorbable form. In this study, respondents consuming vitamin C “fairly often” showed an anemia prevalence of 41.2%, compared to 36.4% among those consuming it “occasionally”. Despite minor variations, these data reinforce the role of vitamin C in improving iron bioavailability (19, 22, 23).
Fructose, commonly present in sweet fruits and honey, also demonstrated a positive effect. Respondents with “fairly frequent” fructose intake reported an anemia prevalence of 48.3%, compared to 43.3% among those consuming it “occasionally”. While the trend was less consistent, literature indicates that fructose enhances iron solubility through the formation of soluble complexes, thereby supporting absorption (24-26).
Taken together, these findings confirm that regular consumption of dietary enhancers — including heme iron, non-heme iron, vitamin C, and fructose — plays a pivotal role in reducing anemia prevalence among adolescent girls. Nutritional interventions emphasizing enhancer-rich foods may therefore represent an effective strategy to prevent IDA in this population.
5.1. Inhibitors of Iron Absorption
Although nonsignificant, the distribution patterns of iron absorption inhibitors still provide meaningful insights into their potential role in Hb regulation. Tannins, polyphenolic compounds abundant in tea and coffee, are well known to reduce non-heme iron absorption (27). In this study, respondents who consumed tannins “fairly often” and “frequently” had higher anemia prevalence (62.5% and 100%, respectively), although the association was not significant (P = 0.196). These findings align with Yuliasih, who reported that tea consumption near mealtimes significantly reduced Hb levels among adolescent girls (28). Similarly, Olchowik-Grabarek et al. demonstrated that tannins can inhibit iron absorption by up to 60%, depending on dosage and timing (29). Mechanistically, tannins form insoluble complexes with iron in the gastrointestinal lumen, thereby decreasing bioavailability, particularly for non-heme iron (30).
Polyphenols, widely found in plant-based foods such as spices, cocoa, and herbal teas, also serve as potent inhibitors. In this study, 50% of respondents consuming polyphenols “occasionally” and 46.9% consuming them “fairly often” were anemic, but the relationship was not statistically significant (P = 0.913). The inhibitory effect of polyphenols is highly dependent on their specific type, structure, and the overall dietary composition (31).
Calcium competes with iron for absorption in the small intestine, particularly when consumed in large amounts (> 300 mg per meal). In this study, anemia prevalence was slightly higher among respondents consuming calcium “fairly often” (51.4%) compared to those consuming it “frequently” (42.9%), with no significant association (P = 0.574). These results are consistent with Gleerup et al., who noted that calcium’s inhibitory effect is transient and influenced by its form and dietary interactions (32). Evidence from Bangladesh also suggests avoiding simultaneous consumption of milk or calcium-rich foods with iron sources to minimize absorption interference (33).
Phytates, abundant in staple foods such as rice, wheat, and legumes, strongly chelate iron to form insoluble complexes that hinder absorption. In this study, respondents who consumed phytates “fairly often” had an anemia prevalence of 45.9%, though the association was not significant (P = 0.321). This finding is consistent with (19), who identified phytates as major inhibitors in plant-based diets, especially in developing countries. Strategies such as fermentation, soaking, and germination have been recommended to reduce phytate content and improve iron bioavailability (34).
Overall, while inhibitors did not demonstrate significant statistical associations with anemia status in this study, their potential impact on iron absorption should not be overlooked. The findings highlight the importance of considering not only enhancers but also inhibitors when addressing dietary strategies to prevent IDA in adolescents.
5.2. Conclusions
Although no statistically significant association was observed between the intake of iron absorption inhibitors and anemia status, a higher proportion of anemia was consistently found among adolescents who consumed these inhibitors more frequently. This finding underscores the importance of nutritional education focusing on meal timing and nutrient interactions, particularly among adolescents, who are highly vulnerable to anemia. Strengthening nutrition education programs in schools and families — such as discouraging tea and coffee consumption during mealtimes and promoting food processing techniques that reduce inhibitor levels — may help optimize iron absorption and support anemia prevention strategies in this population.
5.3. Limitations
This study has several limitations. First, its cross-sectional design limits the ability to infer causality between dietary patterns and anemia status. Second, dietary intake was assessed using a self-reported FFQ, which may be subject to recall bias and estimation errors. Third, the study did not include biochemical markers such as serum ferritin or transferrin saturation to confirm iron status. Finally, the relatively small, school-based sample may limit the generalizability of the findings to broader adolescent populations.