1. Background
Correct diagnosis of diseases with nearly identical symptoms is a major challenge in clinical medicine. When multiple diseases present with similar symptoms — varying in number, quality, and intensity — this creates a complex web of information that the human mind cannot fully analyze (1-4). Conventional diagnostic processes may mislead physicians, leading to incorrect initial diagnoses (5-8). On the other hand, ambiguity and uncertainty are inherent in medical science, further complicating the process. Uncertainty arises from subjective patient reports, variable disease manifestations across individuals, and overlapping symptoms among diseases. Therefore, precise, scientific methods are essential to overcome these barriers and facilitate early diagnosis (9-11).
2. Methods
Fuzzy logic-based methods are the best choice for dealing with ambiguity and uncertainty in medical sciences (12). In such methods, numerical values are assigned to human subjective judgments according to their importance (13). Various methods used as accurate, scientific, and efficient tools to speed up the decision-making process are highly interdependent with optimization issues (14-16). Multi-criteria decision-making (MCDM) methods are very useful when the number of decision criteria is high and varied in quality (17, 18). Despite the many approaches presented in this field, the AHP method developed by Thomas Saaty remains the most popular and efficient method (19). The hierarchical analysis method uses the views of experts to form comparative matrices and then derives relative weights from these matrices that represent decision makers’ preferences among the different decision options in terms of decision criteria. The sum of the normalized weights for each option determines the total weight of each option, which helps the decision maker to choose the best option. In this paper, we use the fuzzy hierarchical analysis (FAHP) method proposed by Chang (20) to select the best disease option from among those with nearly identical symptoms (21). We first identified the symptoms and criteria for the diagnosis of the three diseases discussed in this study by referring to a specialist physician and medical books. Then, using Chang’s (14, 20, 22, 23) FAHP process, we form comparative matrices and determine the weights of symptoms relative to each other and to the three diseases. Finally, by ranking disease options, we prioritize these three diseases and choose the best disease option. It is important to note that the initial diagnosis in this study is performed prior to any time-consuming and costly tests and is on the patient’s clinical examination and symptoms. In fact, this process helps the physician choose the right path for diagnosis and treatment of the disease without losing the golden opportunity. Therefore, with this initial diagnosis and based on fuzzy mathematical priority setting, the physician begins treatment and medical procedures and then confirms the diagnosis by performing specialized tests and examinations, thereby avoiding wasted time on trial and error among different diseases (1, 24-27).
3. Results
Ranking of diseases by FAHP method: In this section, we attempt to differentiate between Crimean-Congo hemorrhagic fever (CCHF), bacterial meningitis, and severe influenza using the FAHP method (28). The selection of CCHF, bacterial meningitis, and influenza was based on the recommendation of an infectious disease specialist, with an emphasis on diseases that commonly present with overlapping symptoms and are often misdiagnosed. Although the model was applied to these three conditions, the primary aim of this study is to demonstrate the diagnostic applicability of the FAHP framework. Once validated, this method can be easily extended to include other diseases with similar diagnostic challenges. As noted earlier, according to the specialist, the symptoms of these three diseases are almost identical, and 16 clinical signs have been identified to distinguish between these three diseases. The selected 16 clinical symptoms were determined based on WHO’s case definition for CCHF and CDC guidelines for the diagnosis of bacterial meningitis, in addition to expert opinion. These symptoms were chosen because they are commonly observable during initial clinical examination and do not rely on laboratory results. In fact, these 16 indicators constitute the decision criteria in the fuzzy AHP method which we represent using symbols C1, C2 … and C16 to represent them. In the first step, based on expert information, we compared the different symptoms in pairs. For this purpose, we initially consulted three infectious disease specialists. The 16 selected symptoms were pairwise compared in terms of importance for differential diagnosis, based on their expert medical judgment. To synthesize the individual assessments and ensure consistency, we used the geometric mean method to aggregate the fuzzy pairwise comparison values. This approach is a well-established technique in fuzzy multi-criteria decision-making (FMCDM) and FAHP, providing a mathematically robust way to combine multiple expert opinions while minimizing individual bias and subjectivity. The results of a clinical examination of a patient with CCHF were used. Then, we formulate the comparison matrices based on Chang’s FAHP (20). It is important to note that the values used in the pairwise comparison tables between diseases and symptoms are fuzzy triangular numbers derived from the 9-point Saaty Scale (29). Specifically, the fuzzy number 1 represents equal importance between two symptoms, while the fuzzy number 9 indicates absolute dominance of one symptom over another. Intermediate importance levels are represented by fuzzy numbers between 1 and 9. The pairwise comparisons of 16 clinical signs of the patient’s symptoms are conducted in the first step. In the second step of the fuzzy analytic hierarchy process (FAHP) for the diagnosis, we compare each of the three diseases under each of the 16 symptoms separately. The results of this comparison are shown in Tables 1 - 4. To enhance the readability of the mathematically dense Tables 1 - 4, a descriptive summary of the observed trends in symptom prioritization across the three diseases is provided. Based on the FAHP analysis: The CCHF received higher weightings in symptoms related to bleeding, sweating, retro-orbital pain, and complexion changes, aligning with its typical hemorrhagic profile. Bacterial meningitis was strongly associated with neck stiffness, level of consciousness disturbances, and convulsion, which are recognized neurological markers of meningitis. Severe influenza showed higher scores in cough, sore throat, and body pain, reflecting the classical respiratory and systemic symptoms of influenza. Other symptoms such as fever, headache, nausea, and vomiting received moderate weightings and were shared among all three diseases with close values, due to their general, non-specific nature.
| Variables | CCHF | Bacterial Meningitis | Severe Flu |
|---|---|---|---|
| Fever | |||
| CCHF | (1 1 1) | (1/4 1/3 1/2) | (1/7 1/6 1/5) |
| Bacterial meningitis | (2 3 4) | (1 1 1) | (1/5 1/4 1/3) |
| Severe flu | (5 6 7) | (3 4 5) | (1 1 1) |
| Headache | |||
| CCHF | (1 1 1) | (1/7 1/6 1/5) | (1/5 1/4 1/3) |
| Bacterial meningitis | (5 6 7) | (1 1 1) | (2 3 4) |
| Severe flu | (3 4 5) | (1/4 1/3 1/2) | (1 1 1) |
| Nausea | |||
| CCHF | (1 1 1) | (1/7 1/6 1/5) | (1/5 1/4 1/3) |
| Bacterial meningitis | (5 6 7) | (1 1 1) | (2 3 4) |
| Severe flu | (3 4 5) | (1/4 1/3 1/2) | (1 1 1) |
| Vomiting | |||
| CCHF | (1 1 1) | (5 6 7) | (2 3 4) |
| Bacterial meningitis | (1/7 1/6 1/5) | (1 1 1) | (1/5 1/4 1/3) |
| Severe flu | (1/4 1/3 1/2) | (3 4 5) | (1 1 1) |
Abbreviation: CCHF, Crimean-Congo hemorrhagic fever.
| Variables | CCHF | Bacterial Meningitis | Severe Flu |
|---|---|---|---|
| Diarrhea | |||
| CCHF | (1 1 1) | (4 5 6) | (1 1 1) |
| Bacterial meningitis | (1/6 1/5 1/4) | (1 1 1) | (1/6 1/5 1/4) |
| Severe flu | (1 1 1) | (4 5 6) | (1 1 1) |
| Sweating | |||
| CCHF | (1 1 1) | (1/5 1/4 1/3) | (1/8 1/7 1/6) |
| Bacterial meningitis | (3 4 5) | (1 1 1) | (1/5 1/4 1/3) |
| Severe flu | (6 7 8) | (3 4 5) | (1 1 1) |
| Body pain | |||
| CCHF | (1 1 1) | (3 4 5) | (1/5 1/4 1/3) |
| Bacterial meningitis | (1/5 1/4 1/3) | (1 1 1) | (1/8 1/7 1/6) |
| Severe flu | (3 4 5) | (6 7 8) | (1 1 1) |
| Sore throat | |||
| CCHF | (1 1 1) | (2 3 4) | (1/6 1/5 1/4) |
| Bacterial meningitis | (1/4 1/3 1/2) | (1 1 1) | (1/8 1/7 1/6) |
| Severe flu | (4 5 6) | (6 7 8) | (1 1 1) |
Abbreviation: CCHF, Crimean-Congo hemorrhagic fever.
| Variables | CCHF | Bacterial Meningitis | Severe Flu |
|---|---|---|---|
| Bleeding | |||
| CCHF | (1 1 1) | (5 6 7) | (6 7 8) |
| Bacterial meningitis | (1/7 1/6 1/5) | (1 1 1) | (1 2 3) |
| Severe flu | (1/8 1/7 1/6) | (1/3 1/2 1) | (1 1 1) |
| Convulsion | |||
| CCHF | (1 1 1) | (1/9 1/8 1/7) | (1 2 3) |
| Bacterial meningitis | (7 8 9) | (1 1 1) | (9 9 9) |
| Severe flu | (1/3 1/2 1) | (1/9 1/9 1/9) | (1 1 1) |
| Cough | |||
| CCHF | (1 1 1) | (1/5 1/4 1/3) | (1/8 1/7 1/6) |
| Bacterial meningitis | (3 4 5) | (1 1 1) | (1/5 1/4 1/3) |
| Severe flu | (6 7 8) | (3 4 5) | (1 1 1) |
| Level of consciousness | |||
| CCHF | (1 1 1) | (4 5 6) | (2 3 4) |
| Bacterial meningitis | (1/6 1/5 1/4) | (1 1 1) | (1/4 1/3 1/2) |
| Severe flu | (1/4 1/3 1/2) | (2 3 4) | (1 1 1) |
Abbreviation: CCHF, Crimean-Congo hemorrhagic fever.
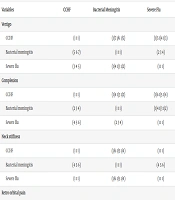
| Variables | CCHF | Bacterial Meningitis | Severe Flu |
|---|---|---|---|
| Vertigo | |||
| CCHF | (1 1 1) | (1/7 1/6 1/5) | (1/5 1/4 1/3) |
| Bacterial meningitis | (5 6 7) | (1 1 1) | (2 3 4) |
| Severe flu | (3 4 5) | (1/4 1/3 1/2) | (1 1 1) |
| Complexion | |||
| CCHF | (1 1 1) | (1/4 1/3 1/2) | (1/6 1/5 1/4) |
| Bacterial meningitis | (2 3 4) | (1 1 1) | (1/4 1/3 1/2) |
| Severe flu | (4 5 6) | (2 3 4) | (1 1 1) |
| Neck stiffness | |||
| CCHF | (1 1 1) | (1/6 1/5 1/4) | (1 1 1) |
| Bacterial meningitis | (4 5 6) | (1 1 1) | (4 5 6) |
| Severe flu | (1 1 1) | (1/6 1/5 1/4) | (1 1 1) |
| Retro orbital pain | |||
| CCHF | (1 1 1) | (3 4 5) | (5 6 7) |
| Bacterial meningitis | (1/5 1/4 1/3) | (1 1 1) | (2 3 4) |
| Severe flu | (1/7 1/6 1/5) | (1/4 1/3 1/2) | (1 1 1) |
Abbreviation: CCHF, Crimean-Congo hemorrhagic fever.
Now we calculate the weights of the three diseases relative to the symptoms. We calculated the inter-rater agreement using Cohen’s kappa coefficient. The value obtained was κ = 0.82, indicating substantial agreement among the three infectious disease specialists regarding the symptom comparisons. The results are shown in Table 5. Finally, we multiply the weight of each disease under each symptom by the corresponding symptom weight and add up the resulting numbers. This will determine the final score for each disease. The results are shown in Table 5.
| Variables | CCHF | Bacterial Meningitis | Severe Flu |
|---|---|---|---|
| WC1 | 0.2806 | 0.2806 | 0.4389 |
| WC2 | 0.2422 | 0.3789 | 0.3789 |
| WC3 | 0.2422 | 0.3789 | 0.3789 |
| WC4 | 0.3789 | 0.2422 | 0.3789 |
| WC5 | 0.3789 | 0.2422 | 0.3789 |
| WC6 | 0.2806 | 0.2806 | 0.4389 |
| WC7 | 0.2806 | 0.2806 | 0.4389 |
| WC8 | 0.2806 | 0.2806 | 0.4389 |
| WC9 | 0.2998 | 0.2998 | 0.4003 |
| WC10 | 0.2998 | 0.2998 | 0.4003 |
| WC11 | 0.2806 | 0.2806 | 0.4389 |
| WC12 | 0.3789 | 0.2422 | 0.3789 |
| WC13 | 0.2422 | 0.3789 | 0.3789 |
| WC14 | 0.2806 | 0.2806 | 0.4389 |
| WC15 | 0.3333 | 0.3333 | 0.3333 |
| WC16 | 0.2998 | 0.2998 | 0.4003 |
| Final score of diseases | 0.3584 | 0.3120 | 0.3296 |
Abbreviation: CCHF, Crimean-Congo hemorrhagic fever.
4. Discussion
To further clarify the differentiation process, it is important to emphasize how the fuzzy AHP method provides a systematic comparison of clinical symptoms that are often ambiguous and overlapping among the three diseases. For instance, while fever and headache are common to all three, the method evaluates their relative diagnostic significance through expert-derived weights. The CCHF tends to score higher in criteria like bleeding and retro-orbital pain, whereas bacterial meningitis receives higher weights in symptoms like neck stiffness and level of consciousness impairment. Severe influenza is more strongly associated with cough, sore throat, and generalized body pain. By incorporating these weighted assessments into a hierarchical model, the fuzzy AHP approach enables a prioritized differential diagnosis based solely on clinical examination. This is particularly valuable during the early stages of patient presentation when laboratory results are not yet available, allowing physicians to initiate timely and appropriate management. It is important to note that the proposed fuzzy prioritization system is intended to support, not replace, the clinical decision-making process. As shown in Table 5, in some cases the final prioritization scores may be very close — for example, the difference between CCHF and severe influenza was only 0.03. In such situations, no absolute threshold was predefined, as the system is intended to assist physicians in forming a quicker diagnostic hypothesis, not to provide definitive diagnosis. When prioritization scores are very close, the final judgment should rely on the clinician’s broader assessment, including additional clinical signs, patient history, epidemiological context, and possibly repeating the evaluation process with adjusted inputs. This flexibility is an inherent advantage of decision support systems based on fuzzy logic. In some cases, the weights calculated through the FAHP may not align with traditional clinical expectations. This is not necessarily an error but rather a result of aggregating expert judgments within a fuzzy mathematical framework. Since symptoms can present with overlapping features across multiple diseases, the relative importance of each symptom may appear evenly distributed — even for those typically regarded as pathognomonic. For example, neck stiffness, though often associated with meningitis, can also occur in other infectious conditions. Our model reflects this ambiguity, aiming to support, not override, clinical decision-making. This framework, although applied to three diseases in the current study, is inherently scalable. By accessing retrospective clinical data from patients with confirmed diagnoses of additional diseases — such as dengue, malaria, or COVID-19 — and through collaboration with specialists in those respective fields, the model can be re-applied for broader differential diagnosis purposes. Such an approach would allow the methodology to be adapted to other disease clusters where symptom overlap presents a clinical challenge, thereby enhancing its practical utility in diverse healthcare settings. A practical demonstration of this type of methodology was already implemented in our previous work (9), where a mobile application based on that model was developed and tested by several infectious disease specialists. Although the dataset used in this study has been previously employed in an earlier publication (9), that work was based on an entirely different analytical framework involving custom-designed linear algebra and optimization techniques. In contrast, the present study applies a standard FAHP methodology. The novelty of this work lies in the adaptation of FAHP to differential diagnosis and the incorporation of expert-driven symptom weighting in a fuzzy decision-making context. According to user feedback, the app achieved approximately 85% accuracy in real-world diagnoses. A similar strategy can be applied to the current FAHP-based model. Given the structure of the method, it can be translated into a rule-based or fuzzy logic-based decision-support tool, potentially deployable as a mobile app or an integrated feature within electronic health record (EHR) systems. Such applications could assist clinicians, especially in emergency or low-resource settings, where rapid differential diagnosis is essential. This study presents a novel application of the FAHP to support the differential diagnosis of infectious diseases with overlapping clinical presentations. A key strength lies in its integration of expert clinical judgment with a robust MCDM framework, enhancing diagnostic precision, particularly in resource-limited settings where laboratory confirmation may be delayed or unavailable. Moreover, the method’s transparency and adaptability make it suitable for extension to other disease clusters, offering potential scalability for broader clinical applications, such as incorporating diseases like dengue or COVID-19. However, several limitations must be acknowledged. First, the model was applied to a limited set of three diseases and 16 symptoms; expanding the framework to include a broader range of differential diagnoses, such as other viral hemorrhagic fevers, will require further validation and input from specialists. Second, the reliance on subjective expert evaluations, despite objective aggregation using the geometric mean, may introduce bias, as seen in the close prioritization scores (e.g., 0.03 difference between CCHF and influenza in Table 5). This model is designed to prioritize the likelihood of differential diagnoses based on symptom importance, rather than to provide a final diagnosis. The output scores assist clinicians by highlighting the most probable condition among similar diseases. Even small differences in scores may have practical value in guiding the sequence of diagnostic testing or empirical treatment decisions. Therefore, we do not impose a strict cutoff for significance, leaving the final interpretation to clinical judgment. Third, the absence of real-world patient testing in this study limits the assessment of diagnostic accuracy metrics, including sensitivity and specificity. This limitation is partially mitigated by prior work achieving approximately 85% accuracy in real-world diagnoses (9). Future studies will validate the model with patient cohorts to quantify these metrics. In recent years, machine learning and artificial intelligence (AI)-based models such as random forests, support vector machines, and deep neural networks have been widely applied to infectious disease diagnosis. These models often require large volumes of labeled data and complex training pipelines. In contrast, FAHP offers a transparent, expert-driven alternative that does not depend on large datasets and is particularly suited for resource-limited settings. While AI models may achieve high accuracy in classification tasks, they often lack interpretability, making them less accessible for clinicians. The FAHP allows integration of clinical reasoning into the diagnostic process and facilitates consensus among domain experts. Therefore, the method serves as a complementary tool that bridges the gap between clinical expertise and decision support systems.
4.1. Conclusions
Accurate diagnosis is one of the most essential components of effective medical intervention. Prompt identification of a disease based on clinical findings — without losing the critical window for treatment — is vital, as diagnostic delays can lead to severe outcomes. However, differentiating between diseases with nearly identical symptoms remains a major clinical challenge. In this study, we developed a FMCDM and prioritization model to support physicians in minimizing early diagnostic errors. Three infectious diseases — CCHF, bacterial meningitis, and severe influenza — were selected as case examples due to their similar clinical presentations. Diagnostic criteria were defined using reliable references and expert opinions, and the diseases were ranked according to their likelihood based on weighted symptom analysis. The results demonstrate that the FAHP-based approach can provide structured and transparent support for differential diagnosis, potentially improving the accuracy and efficiency of clinical decision-making. Further refinement of symptom weighting and validation with larger clinical datasets may