1. Background
Tuberculosis (TB), caused by Mycobacterium tuberculosis, remains a major global health challenge, predominantly affecting the lungs as pulmonary tuberculosis (PTB), though extrapulmonary manifestations are also common (1). Despite extensive international control efforts, TB remains endemic in countries such as Iran, contributing substantially to the global disease burden, with an estimated one-quarter of the world’s population infected either latently or actively (2, 3). The PTB typically presents with nonspecific respiratory symptoms such as persistent cough, fever, dyspnea, wheezing, lymphadenopathy, and weight loss, making timely clinical diagnosis difficult (4). Early and accurate diagnosis of PTB is essential to initiate appropriate treatment and interrupt disease transmission.
Conventional diagnostic methods, including sputum smear microscopy for acid-fast bacilli (AFB), culture, and molecular assays such as Bacillus Koch polymerase chain reaction (BK-PCR), are considered the gold standard but suffer from limitations such as delayed results and reduced sensitivity, especially in smear-negative or paucibacillary cases (4). Immunological tests like the tuberculin skin test and interferon-gamma release assays (IGRA) provide supplementary information but cannot reliably distinguish active from latent infection (4). Chest radiography is commonly used for initial assessment but often lacks sufficient sensitivity and specificity in early or atypical PTB presentations (3, 4).
Chest CT has emerged as a valuable imaging modality capable of detecting subtle pulmonary abnormalities not visible on standard radiographs. Typical CT features of active PTB include areas of consolidation caused by airway obstruction from inflammatory exudates (5), cavitary lesions that strongly correlate with disease activity, ground-glass opacities (GGOs), centrilobular nodules (small 5 - 10 mm nodules located centrally within secondary pulmonary lobules), and the tree-in-bud pattern indicative of endobronchial spread (6, 7). Additional radiologic signs such as pleural effusions (8), empyema with loculated collections, and lymphadenopathy with central necrosis in hilar and mediastinal nodes further support diagnosis (9).
Despite these advantages, chest CT is not routinely integrated into national TB diagnostic algorithms in many countries (10). Given the limitations of conventional microbiological methods, particularly their time-consuming nature and lower sensitivity in smear-negative or paucibacillary cases, chest CT holds significant potential as a complementary diagnostic tool.
2. Objectives
The present study aims to evaluate the diagnostic accuracy of unenhanced chest CT in identifying active PTB among suspected cases and to assess the correlation between CT findings and microbiological results from culture and BK-PCR. By providing evidence to support the integration of CT imaging into diagnostic protocols, this research seeks to improve early detection and diagnostic precision, ultimately enhancing clinical management and control efforts for PTB in endemic settings worldwide.
3. Methods
This cross-sectional diagnostic accuracy study was conducted to evaluate the utility of unenhanced chest CT in diagnosing active PTB among suspected cases. The research took place at the Radiology Department of Bu-Ali-Sina Hospital, affiliated with Zahedan University of Medical Sciences (ZAUMS), Iran, over a six-year period from March 2014 to November 2020. Consecutive enrollment was applied to minimize selection bias.
3.1. Study Population and Eligibility Criteria
A total of 130 patients, aged 15 to 80 years, who were clinically suspected of having PTB and admitted during the study period, were prospectively enrolled. Clinical suspicion was standardized as the presence of at least one key symptom (persistent cough > 2 weeks, fever, weight loss, hemoptysis, or night sweats) combined with abnormal chest auscultation or radiographic suspicion. Eligibility required that patients underwent chest CT and had at least one microbiological test performed, including AFB smear, culture, or BK-PCR. Patients with a history of prior TB treatment, chronic pulmonary diseases (e.g., COPD, bronchiectasis), immunocompromised conditions (HIV/AIDS, malignancy, diabetes), or incomplete diagnostic data were excluded. The sample size was determined based on hospital admission rates for suspected PTB within the study period, with a power analysis indicating that a minimum of 120 participants was necessary to detect a 15% difference in diagnostic sensitivity between CT and microbiological testing, achieving 80% power at α = 0.05.
3.1.1. Inclusion Criteria
Participants eligible for inclusion in the study were individuals aged between 15 and 80 years who presented with clinical suspicion of PTB. All included patients were required to have undergone chest CT imaging as well as at least one microbiological diagnostic test, such as AFB smear, culture, or BK-PCR.
3.1.2. Exclusion Criteria
The study excluded individuals with a known history of TB or those who had received prior anti-TB treatment. Additional exclusion criteria included the presence of chronic pulmonary diseases (e.g., chronic obstructive pulmonary disease or bronchiectasis), immunocompromising conditions such as HIV/AIDS, diabetes mellitus, malignancy, or those receiving immunosuppressive therapy. Patients with comorbidities that could confound radiologic interpretations, such as renal failure, hepatitis, or uncontrolled hypertension, were also excluded. These exclusions were applied to minimize confounding and allow for a clearer assessment of 'pure PTB'. However, this limits generalizability to real-world TB populations where comorbidities such as HIV or diabetes are common. Pregnant women and patients with incomplete diagnostic data, including missing CT imaging or microbiological test results, were not included in the study.
3.2. Diagnostic Reference Standards and Patient Grouping
All participants underwent a standardized diagnostic workup for PTB, comprising microbiological and radiologic assessments. Microbiological confirmation, serving as the reference standard, was defined as either a positive culture for M. tuberculosis or a positive BK-PCR result from sputum or bronchoalveolar lavage specimens. Active PTB was diagnosed based on compatible clinical presentation combined with either positive microbiological confirmation or characteristic CT findings (cavitation, tree-in-bud pattern, centrilobular nodules) in the absence of alternative diagnoses. For subgroup analysis, patients were classified by AFB smear results using the Ziehl-Neelsen method, with smear-positive cases stratified into +1, +2, or +3 grades and smear-negative cases showing no detectable bacilli. The primary outcome was the diagnostic accuracy of unenhanced chest CT in detecting microbiologically confirmed PTB, while exposures included demographic characteristics and clinical symptoms. Predefined CT findings served as predictors, and potential confounders such as prior lung disease, immunosuppression, or incomplete microbiological testing were controlled through strict exclusion criteria, with no significant effect modifiers identified.
3.3. Chest Computed Tomography Acquisition and Interpretation
All patients underwent non-contrast spiral chest CT imaging using a 16-slice multi-detector CT scanner (Siemens, Somatom Emotion). Images were acquired in the axial plane with a slice thickness of 5 mm, without intravenous contrast, to maintain standardization and minimize patient risk. The CT images were reviewed retrospectively by a board-certified thoracic radiologist and cross-validated with a senior expert radiologist. Inter-observer agreement was measured using Cohen’s Kappa statistic, yielding a coefficient of 0.87, indicating excellent concordance.
Eight predefined CT features were evaluated systematically: Cavitation, centrilobular nodules, tree-in-bud pattern, consolidation, GGO, lymphadenopathy, pleural effusion, and empyema. Among these, centrilobular nodules, tree-in-bud appearance, and cavitation were designated as key diagnostic indicators of active PTB and were analyzed in detail to assess their diagnostic performance. The CT images were interpreted independently, and radiologists were blinded to microbiological results to minimize diagnostic review bias.
3.4. Data Collection Procedure
Demographic data (age and sex), clinical symptoms, AFB smear results, culture/PCR findings, and CT scan interpretations were systematically extracted from patients’ electronic medical records. All CT images were reviewed using the hospital’s picture archiving and communication system (PACS) by two blinded thoracic radiologists, achieving excellent inter-observer agreement (Cohen’s Kappa = 0.87). To minimize bias, patients were consecutively included, and those with confounding comorbidities were excluded to reduce the risk of diagnostic misclassification.
3.5. Ethical Approval
Written informed consent was obtained from all participants prior to their enrollment in the study. The study was reviewed and approved by the Ethics Committee of ZAUMS (IR.ZAUMS.REC.1398.303). The research was conducted in accordance with the ethical standards of the Declaration of Helsinki.
3.6. Statistical Analysis
Data were analyzed using SPSS 26.0. Descriptive statistics, including frequencies and percentages, were used to summarize categorical variables. Diagnostic accuracy metrics — sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Youden Index, and likelihood ratios (LRs) — were calculated for the three key CT findings considered indicative of active PTB: Centrilobular nodules, tree-in-bud appearance, and cavitation. Associations between CT findings and microbiologically confirmed TB diagnosis were assessed using the chi-square test. A P-value < 0.05 was considered statistically significant.
The Youden Index is a summary measure of the effectiveness of a diagnostic test. It combines both sensitivity and specificity into a single number to assess the overall discriminative power of a test. The Youden Index ranges from -1 to 1, but in practical diagnostic testing, it typically falls between 0 and 1. A value of 0 indicates that the test performs no better than random chance. A value close to 1 reflects excellent diagnostic performance, with both high sensitivity and specificity. Negative values (< 0), though rare, suggest the test is misleading, classifying non-diseased individuals as diseased or vice versa. In clinical research, a higher Youden Index indicates a better balance between correctly identifying true positives and true negatives, making it useful for selecting optimal cut-off points or comparing the diagnostic accuracy of different methods.
4. Results
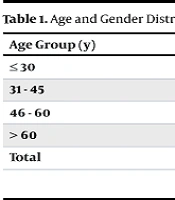
A total of 130 patients suspected of having PTB were included in the study, with a mean age of 53.12 years. Among them, 50.8% were male and 49.2% were female. The patients were categorized into four age groups, as shown in Table 1. Table 2 illustrates the frequency of various CT imaging findings in patients with suspected PTB, along with their relationship to smear grades (+1, +2, +3, and smear-negative) and microbiological confirmation (culture/PCR).
| Age Group (y) | Male | Female | Total |
|---|---|---|---|
| ≤ 30 | 16 (57) | 12 (43) | 28 (21.5) |
| 31 - 45 | 11 (65) | 6 (35) | 17 (13.1) |
| 46 - 60 | 13 (48) | 14 (52) | 27 (20.8) |
| > 60 | 24 (41) | 34 (59) | 58 (44.6) |
| Total | 64 (49.2) | 66 (50.8) | 130 |
a Values are expressed as No. (%).
| CT Findings | Total | +1 | +2 | +3 | Smear Negative | Culture/PCR Positive | Culture/PCR Negative |
|---|---|---|---|---|---|---|---|
| Cavitation | 59 (45.4) | 12 (20.3) | 25 (42.4) | 17 (28.8) | 5 (8.5) | 27 (45.8) | 32 (54.2) |
| Tree-in-bud | 64 (49.2) | 18 (28.1) | 26 (40.6) | 14 (21.9) | 6 (9.4) | 32 (50) | 32 (50) |
| Nodule | 80 (61.5) | 20 (25.0) | 35 (43.8) | 20 (25.0) | 5 (6.3) | 39 (48.8) | 41 (51.2) |
| GGO | 44 (33.8) | 18 (40.9) | 13 (29.5) | 5 (11.4) | 8 (18.2) | 10 (22.7) | 34 (77.3) |
| Consolidation | 95 (73.1) | 37 (38.9) | 27 (28.4) | 18 (18.9) | 13 (13.7) | 33 (34.7) | 62 (65.3) |
| Pleural effusion | 26 (20.0) | 11 (42.3) | 5 (19.2) | 5 (19.2) | 5 (19.2) | 5 (19.2) | 21 (81.7) |
| Empyema | 8 (6.2) | 4 (50.0) | 3 (37.5) | 1 (12.5) | 0 (0.0) | 3 (37.5) | 5 (62.5) |
| Lymphadenopathy | 62 (47.7) | 22 (35.5) | 19 (30.6) | 12 (19.4) | 9 (14.5) | 20 (32.3) | 42 (67.7) |
| Certain findings b | 30 (23.1) | 3 (10) | 13 (43.3) | 11 (36.7) | 3 (10) | 17 (56.7) | 13 (43.3) |
Abbreviations: CT, computed tomography; GGO, ground-glass opacity.
a Values are expressed as No. (%).
b Presence of any of the following: Cavitation, tree-in-bud, or nodules.
The analysis of CT imaging findings in suspected PTB revealed that consolidation (73.1%) was the most common abnormality, followed by nodules (61.5%), tree-in-bud appearance (49.2%), and cavitation (45.4%), with cavitation frequently associated with higher smear grades (+2 and +3). Less frequent findings included GGO (33.8%), pleural effusion (20.0%), and empyema (6.2%). Correlation with microbiological confirmation showed that cavitation, tree-in-bud, and certain hallmark findings were more often linked to culture/PCR positivity, suggesting a stronger association with active TB, while GGO, pleural effusion, and lymphadenopathy were predominantly seen in culture/PCR-negative cases, likely reflecting early-stage disease or non-TB etiologies. Overall, these results highlight that CT imaging, particularly when demonstrating classic TB patterns, serves as an important diagnostic adjunct that complements microbiological testing in the evaluation of PTB.
Table 3 presents the diagnostic accuracy metrics of different AFB smear grades (+1, +2, +3, and smear-negative) in relation to culture/PCR-confirmed PTB. The analysis shows that the +2 AFB smear grade had the highest sensitivity (81.3%) and the greatest Youden Index (0.28), indicating it offers the best balance between detecting true positives and minimizing false negatives. Although the smear-negative group demonstrated the highest specificity (86.7%) and the highest NPV (81.3%), its low sensitivity (25.0%) limits its diagnostic reliability for confirming TB. In terms of predictive values, the +1 smear grade had the highest PPV (56.7%) and also achieved the highest LR (2.5), closely followed by the +2 smear (2.49). Overall, these findings suggest that the +2 AFB smear provides the most effective diagnostic performance for predicting culture/PCR-confirmed PTB.
| AFB Smear Group | P-Value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Youden Index | LR |
|---|---|---|---|---|---|---|---|
| +1 Positive | 0.20 | 58.70 | 62.00 | 56.70 | 71.00 | 0.12 | 2.5 |
| +2 Positive | 0.02 | 81.30 | 47.40 | 52.90 | 77.30 | 0.28 | 2.49 |
| +3 Positive | 0.23 | 46.20 | 72.40 | 49.20 | 75.00 | 0.18 | 1.64 |
| Smear Negative | 0.57 | 25.00 | 86.70 | 33.30 | 81.30 | 0.11 | 1.78 |
Abbreviations: AFB, acid-fast bacilli; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
5. Discussion
This cross-sectional six-year study evaluated the diagnostic performance of unenhanced chest CT in suspected PTB cases, with a particular focus on hallmark radiological findings and their correlation with smear grades and microbiological confirmation. The study results indicated that consolidation was the most common CT finding in suspected PTB, followed by centrilobular nodules, tree-in-bud appearance, and cavitation. While consolidation was frequent, it lacked specificity, as it may also occur in other pulmonary infections and inflammatory conditions. In contrast, the tree-in-bud pattern, centrilobular nodules, and cavitation were strongly associated with active PTB and culture/PCR positivity.
These hallmark features reflect underlying pathological mechanisms: The tree-in-bud pattern is indicative of endobronchial spread of infection, while cavitation results from caseous necrosis and is associated with a high bacillary burden, thus correlating with higher smear grades. The frequent detection of centrilobular nodules alongside the tree-in-bud pattern suggests small airway involvement, characteristic of active disease. These findings are consistent with previous studies that have highlighted these CT patterns as radiologic hallmarks of active PTB and important predictors of microbiological confirmation (11-15).
In contrast, GGO, pleural effusion, and lymphadenopathy were predominantly observed in culture/PCR-negative patients in this cohort. These findings likely represent early-stage disease or non-tuberculous etiologies, as also noted in earlier reports (11, 12). Therefore, the diagnostic interpretation of CT in suspected PTB should place greater emphasis on hallmark patterns such as tree-in-bud, cavitation, and centrilobular nodules, as they more reliably indicate active TB.
The analysis of CT findings in relation to AFB smear grades demonstrated that cavitation and tree-in-bud patterns were strongly correlated with higher smear grades (+2 and +3) and culture/PCR positivity. These findings align with previous research indicating that these CT features are strongly associated with active PTB and higher bacillary loads (11-15). In this study, the +2 smear grade showed the highest sensitivity (81.3%) and the best diagnostic balance (Youden Index 0.28), making it the most reliable predictor of microbiologically confirmed TB when combined with CT findings. The +1 smear grade, although associated with a lower sensitivity, had the highest PPV (56.7%) and a LR comparable to the +2 group.
Conversely, the smear-negative group, despite having the highest specificity (86.7%) and a high NPV (81.3%), demonstrated poor sensitivity (25%), limiting its diagnostic utility. These findings highlight the pivotal role of CT imaging in enhancing diagnostic accuracy, particularly in patients with smear-negative or intermediate smear grades (+1, +2), where microbiological evidence may be inconclusive. Similar to previous reports demonstrating high CT sensitivity and specificity in both smear-positive and smear-negative cases (11, 13), our results confirm that CT provides valuable diagnostic support. Therefore, incorporating CT findings alongside smear microscopy and culture results can substantially improve early detection and facilitate timely initiation of treatment in suspected PTB cases.
The diagnostic accuracy of CT observed in this study is consistent with previous reports. For example, a Pakistani study documented sensitivity and specificity rates of 81% and 85%, respectively, supporting CT’s utility in early detection (11). Similarly, an Indian cohort study found sensitivity rates of 89% and specificity of 79.25% in smear-positive cases, and 88.5% sensitivity with 84.6% specificity in smear-negative cases (12). These findings emphasize that CT retains high diagnostic value even when smear microscopy is negative. Furthermore, an Indonesian study using a structured CT scoring system reported an impressive diagnostic accuracy of 95.1% (13), suggesting that incorporating scoring methods may further enhance diagnostic precision. Iranian studies also reported sensitivities exceeding 95% and NPVs above 90%, reinforcing CT’s reliability in detecting active PTB (14).
Compared with these studies, the current research focused on specific hallmark signs without using a formal scoring system, yet achieved comparable diagnostic outcomes. This demonstrates that targeted interpretation of CT features, particularly tree-in-bud, cavitation, and centrilobular nodules, can significantly improve diagnostic accuracy even without complex scoring. Delayed diagnosis remains a major obstacle to TB control, particularly in endemic regions. Alavi et al. (15) reported an average diagnostic delay of 73 days, affecting 65.5% of patients, with significant predictors including female sex, smoking, and immunosuppressive drug use. Similarly, Neshati et al. (16) highlighted diagnostic errors, mostly failures in hypothesis generation (72%), as the leading cause of delay. The findings of the current study support the notion that integrating CT into diagnostic workflows, especially for smear-negative and intermediate (+2) cases, can address these gaps by providing timely radiological evidence to initiate treatment earlier.
Khatibi et al. (17) demonstrated the potential of AI-driven tools in improving TB diagnosis. Their two-step decision support system (TPIS) using clinical, radiographic, and laboratory data achieved an area under the curve (AUC) of 92.8% and accuracy of 93.9% in the final diagnosis. While the TPIS utilized structured AI models, the present study focused on direct CT interpretation, showing that even without AI integration, targeted assessment of hallmark features offers substantial diagnostic value.
Despite being considered the gold standard, microbiological methods such as smear microscopy, culture, and PCR have well-documented limitations in paucibacillary or smear-negative PTB. Culture sensitivity decreases significantly in such cases (18), and nucleic acid amplification tests like Xpert MTB/RIF also perform poorly, with sensitivities around 50 - 60% in smear-negative, culture-positive specimens (19). Newer antigen-based tests like NanoDisk-MS have demonstrated higher accuracy in detecting TB, but they are not yet widely accessible (20). In such situations, chest CT scans can play a crucial supporting role, especially when microbiological test results are unclear or inconclusive.
The present findings advocate for integrating unenhanced chest CT into diagnostic algorithms for PTB, particularly in patients with smear-negative or +1/+2 smear results where microbiological confirmation may be delayed or uncertain. For smear-negative patients, hallmark CT signs can support early presumptive therapy, while for +2 cases, CT significantly enhances diagnostic certainty. In +3 smear cases, CT’s diagnostic role is less critical but remains useful for assessing disease extent and complications.
Our findings are further supported by Sharifi Mood et al. (21), who reported a case series of three children developing active PTB despite receiving isoniazid chemoprophylaxis after exposure to a smear-positive case. Their study demonstrated that, even with proper prophylactic regimens, active PTB may still occur, highlighting the need for continued clinical and radiologic surveillance in high-risk contacts. This reinforces the importance of adjunctive diagnostic tools, such as chest CT, for early detection in similar high-risk populations.
A study conducted by Navid and Keikha (22) investigated a case of misdiagnosed M. abscessus pulmonary infection in Iran. They reported an 85-year-old woman with a prior history of PTB who presented with cough, dyspnea, fever, night sweats, weight loss, hemoptysis, and other systemic symptoms. Based on clinical findings, chest X-ray, and positive AFB smear, she was initially presumed to have reactivated TB and was started on anti-TB therapy. However, her symptoms did not improve. Further microbiological and molecular analyses identified the causative agent as M. abscessus, not M. tuberculosis. Following antibiotic susceptibility testing, the patient was successfully treated with linezolid, amikacin, and cefoxitin. The study found that non-tuberculous mycobacteria (NTM), particularly M. abscessus, can mimic PTB both clinically and radiologically, leading to misdiagnosis and delayed appropriate treatment. The authors emphasized the importance of species-level identification of mycobacteria in suspected TB cases, especially in patients with a history of prior TB.
Studies from Iran and other endemic regions consistently show that CT reduces diagnostic delays and improves early detection (15, 16). Beyond its global relevance, our study has important implications for local and national TB diagnostic strategies. By evaluating CT imaging features in patients without significant comorbidities, our findings provide more precise sensitivity and specificity estimates for 'pure' TB cases. Incorporating these results into Iranian and regional diagnostic algorithms could help optimize the use of imaging resources, prioritize patients most likely to benefit from advanced diagnostics, and improve early detection rates.
Therefore, our study not only contributes to the general understanding of TB imaging but also offers actionable insights for enhancing regional TB care and resource allocation. By focusing on comorbidity-free patients, our findings provide more accurate diagnostic performance estimates that can inform Iranian and regional TB diagnostic algorithms, even if broader generalizability is limited.
5.1. Conclusions
In conclusion, unenhanced chest CT demonstrates significant diagnostic value in detecting active PTB, especially when hallmark features such as tree-in-bud appearance, cavitation, and centrilobular nodules are present. These features strongly correlate with microbiological confirmation and smear positivity, making CT an essential diagnostic adjunct in smear-negative and intermediate smear cases. When integrated with microbiological testing, CT improves early diagnosis and facilitates timely initiation of therapy, thereby contributing to better patient outcomes and TB control, particularly in endemic regions.
Future research could perform subgroup analyses based on age and sex to determine whether diagnostic accuracy or disease characteristics differ across demographic groups. This could help tailor diagnostic criteria or interventions for specific populations. Retrospective evaluation of CT feature scoring may also provide additional insights into disease severity and prognosis. Incorporating a standardized scoring system could enhance the predictive value of imaging studies.
Finally, future studies could utilize logistic regression or other multivariable models to identify independent predictors of disease and to adjust for potential confounders. This approach may improve risk stratification and guide clinical decision-making.
5.2. Strengths and Limitations
Limitations of this study include the single-center design, absence of a structured CT scoring system, and potential selection bias due to the inclusion of only suspected PTB cases. Radiation exposure and cost considerations may limit the generalizability of CT-based screening in low-resource settings. Additionally, we did not perform longitudinal follow-up to assess long-term diagnostic accuracy or treatment response, nor did we perform subgroup analyses by age/sex or apply a structured scoring system. Future studies could address these gaps. Logistic regression models may also help identify independent CT predictors of microbiological confirmation.