1. Background
Thyroid hormones play a critical role in pregnancy as key regulators of fetal growth and neurodevelopment (1, 2). An imbalance in maternal thyroid hormone levels affects pregnancy outcomes (3, 4). Both maternal hypothyroidism and hyperthyroidism can cause developmental disorders in the fetus (5, 6). Thyroid hormones bind to plasma proteins and are delivered to the fetus via the placenta (7). In the third trimester, after the fetal thyroid gland is structurally and functionally mature, the fetus can produce its own thyroid hormones (3).
An increasing body of evidence suggests that exposure to endocrine-disrupting chemicals (EDCs) during pregnancy can affect the thyroid gland function in both mothers and fetuses. These chemicals include air pollutants, various chemicals (8, 9), cadmium (10), and active and passive cigarette smoke (11). However, these studies are limited, and their findings are conflicting. While exposure to pollutants may adversely affect thyroid function, exposure to green space might have beneficial effects. Few studies have evaluated the effect of green space on pregnancy parameters such as gestational diabetes, birth weight, and preterm birth. The results of these studies strongly suggest that exposure to green spaces during pregnancy can affect thyroid function in the fetal and postnatal periods (12, 13). However, these studies did not directly assess the effect of green space on thyroid function in pregnant women or fetuses.
2. Objectives
Conflicting results exist regarding the association of green space and household chemical exposure with thyroid hormone levels in mothers and their fetuses. Therefore, the present study aimed to evaluate the association between household contaminants and green space exposure during pregnancy and thyroid-stimulating hormone (TSH) levels in the cord blood of term newborns.
3. Methods
3.1. Study Population
This cross-sectional study was conducted in Isfahan, the most industrialized and highly polluted city in Iran. A total of 300 pregnant women were randomly selected from public and private clinics. Inclusion criteria were as follows: Term delivery, Iranian nationality, and residence in the study center’s catchment area for at least one year. Women with communication problems were excluded. Mothers with a history of medication use that could influence thyroid hormone secretion during pregnancy, as well as cases with issues related to blood sampling (e.g., insufficient blood sample or inability to measure TSH), were also excluded.
3.2. Ethical Issues
After a complete explanation of the study protocol, written informed consent was obtained from all participants.
3.3. Data Collection
Data were obtained from the PERSIAN birth Cohort Study and included 286 pregnant women who were randomly enrolled. The PERSIAN Birth Cohort Questionnaires were used to collect information on household chemical exposures [including bleach, waxes, glass cleaner, stain liquid, naphthalene, electric air freshener, multi-purpose cleaner, degreased spray, air freshener spray, anti-bug sprays, candles, lute, traditional aromatic incense (Esfand, Peganum harmala), and plastic heating in the microwave oven] and green space exposure (duration of exposure to parks, forests or jungles, farms or gardens, rivers or beaches). These questionnaires were completed at three time points during pregnancy (first, second, and third trimesters). The total exposure to green space (hours) and chemicals (number of exposures) during the nine months of pregnancy was then calculated (14).
For assessment of secondary objectives, childbirth information was collected, including child physical growth parameters (gestational age, birth weight, length, head circumference) and Apgar scores at one and five minutes after birth.
Maternal Body Mass Index (BMI) was categorized as underweight (BMI < 18.5 kg/m2), normal weight (18.5 ≤ BMI < 24.9 kg/m2), overweight (BMI 25 - 29.9 kg/m2), and obese (BMI > 30 kg/m2) (15).
3.4. Biochemical Measurements
For evaluation of cord blood TSH levels, umbilical cord blood samples were obtained from the newborns of participating women. Samples were stored in polypropylene cryovials and kept frozen at -70°C until analysis. The TSH levels were measured using the TSH EIA 96 Test immunoassay kit (Linear Chemicals, Barcelona, Spain, CAT No: 6107225).
3.5. Statistical Analysis
Continuous and categorical variables are presented as mean ± SD, median, interquartile range (IQR), and frequency (%), respectively. The Spearman correlation test was used to evaluate the relationship between TSH level and birth weight, length, head circumference, and Apgar scores. Multiple linear regression was used to assess the relationship between cord TSH level and maternal exposure to household pollutants and green space. The chi-square test was used for comparisons between quartiles of variables. The Kruskal-Wallis test was used to compare TSH levels across exposure quartiles. Assumptions of linearity were verified; future analyses may explore nonlinear associations using polynomial regression. Statistical analyses were performed using the SPSS statistical package (version 18.0, SPSS, Chicago, IL, USA). The significance level was considered as P < 0.05.
4. Results
Data from 286 mothers were completed and analyzed. The characteristics of mothers and their newborns and details of exposures are presented in Table 1. The mean age ± SD of mothers was 29.78 (5.56) years. The mean BMI was 24.57 (6.15) kg/m2, and 12.4% were obese. The mean hours of green space exposure and mean number of chemical exposures during pregnancy were 73.19 hours and 66.15 exposures, respectively.
| Variables | Values | Number of Mothers Having Exposure |
|---|---|---|
| Mothers | ||
| Age (y) | 29.78 ± 5.56 | - |
| BMI (kg/m2) | 24.57 ± 6.15 | - |
| BMI category | ||
| Underweight | 17 (7.8) | - |
| Normal weight | 97 (44.5) | - |
| Overweight | 77 (35.3) | - |
| Obese | 27 (12.4) | - |
| History of thyroid disorders | 44 (18.5) | - |
| Newborns | ||
| Birth weight (gr) | 3151.63 ± 369.64 | - |
| Birth length (cm) | 49.65 ± 3.245 | - |
| Birth head circumference | 34.77 ± 1.93 | - |
| Apgar score | ||
| 1st minute | 8.93 ± 0.50 | - |
| 5th minute | 9.80 ± 0.44 | - |
| TSH (mIU/L) | 8.99 ± 8.50 | - |
| Exposures | ||
| Green space exposure (h) | 73.19 ± 104.62 b | - |
| Pollutants/chemicals exposure (h) | 66.15 ± 107.67 c | - |
| Using chemicals/pollutants | ||
| Bleach | 4.17 ± 6.51 | 127 |
| Waxes | 0.48 ± 2.80 | 9 |
| Glass cleaner | 11.45 ± 27.96 | 96 |
| Stain liquid | 0.63 ± 2.89 | 17 |
| Naphthalene | 0.54 ± 8.03 | 1 |
| Electric air freshener | 6.61 ± 68.97 | 6 |
| Multi-purpose cleaner | 3.39 ± 19.15 | 45 |
| Degreased spray | 2.83 ± 10.63 | 34 |
| Air freshener spray | 6.38 ± 36.55 | 32 |
| Bugs spray | 0.91 ± 8.18 | 16 |
| Candle | 0.71 ± 1.82 | 37 |
| Lute | 1.29 ± 8.47 | 23 |
| Traditional fume (Esfand) | 25.12 ± 47.78 | 174 |
| Plastic heating in the microwave oven | 0.59 ± 8.07 | 2 |
Abbreviations: TSH, thyroid-stimulating hormone; BMI, Body Mass Index.
a Values are expressed as No. (%) or mean ± SD.
b Interquartile range for green space exposure: 74.25 hours.
c Interquartile range for pollutants exposure: 57.75 hours.
The most commonly used pollutants or chemicals were bleach, glass cleaner, and Esfand. The mean hours of exposure were highest for Esfand (25.12 hours), followed by glass cleaner (11.45 hours), electric air freshener (6.61 hours), air freshener spray (6.38 hours), and bleach (4.17 hours). Esfand use was reported by 60.8% of the mothers.
Using the Spearman correlation test, no significant association was found between cord blood TSH level and maternal exposure to pollutants or chemicals or green space.
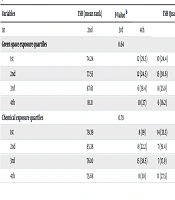
The frequency of mothers in each quartile of TSH levels across quartiles of green space and chemical exposures is presented in Table 2. There was no significant difference in TSH levels between categories of green space exposure or chemical exposure. Similarly, there was no significant difference in the frequency of mothers in each TSH quartile across different exposure quartiles.
| Variables | TSH (Mean Rank) | P-Value b | TSH Quartiles | P-Value c | |||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||||
| Green space exposure quartiles | 0.64 | 0.83 | |||||
| 1st | 74.24 | 12 (29.3) | 10 (24.4) | 11 (26.8) | 8 (19.5) | ||
| 2nd | 77.55 | 12 (24.5) | 15 (30.6) | 10 (20.4) | 12 (24.5) | ||
| 3rd | 87.61 | 6 (19.4) | 8 (25.8) | 7 (22.6) | 10 (32.3) | ||
| 4th | 81.11 | 10 (27) | 6 (16.2) | 12 (32.4) | 9 (24.3) | ||
| Chemical exposure quartiles | 0.78 | 0.08 | |||||
| 1st | 79.39 | 8 (19) | 14 (33.3) | 9 (21.4) | 11 (26.2) | ||
| 2nd | 85.38 | 8 (22.2) | 7 (19.4) | 9 (25.0) | 12 (33.3) | ||
| 3rd | 76.10 | 15 (38.5) | 7 (17.9) | 6 (15.4) | 11 (28.2) | ||
| 4th | 75.68 | 8 (20) | 11 (27.5) | 16 (40.0) | 5 (12.5) | ||
Abbreviation: TSH, thyroid-stimulating hormone.
a Values are expressed as No. (%).
b Data was obtained using the Kruskal-Wallis test.
c Data obtained from the chi-square test.
The association between TSH quartiles and quartiles of green space and pollutants or chemicals was evaluated using multivariate regression models. After adjusting for confounding variables (BMI, newborn gender, and history of thyroid disorders), no significant association was found between these variables and cord blood TSH level [β (95% CI) for green space exposure: -0.02 (-0.08, 0.04), P = 0.51; β (95% CI) for chemical exposure: 0.01 (-0.03, 0.05), P = 0.61]. Sensitivity analyses excluding mothers with a history of thyroid disorders showed consistent null associations (data not shown).
The association between TSH quartiles and the six most commonly used household chemicals is presented in Table 3. No significant association was found between TSH category levels and exposure to bleach, glass cleaner, multi-purpose cleaner, degreased spray, air freshener, or Esfand.
| Chemicals | TSH | P-Value b | |||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||
| Bleach | 24 (25.8) | 22 (23.7) | 26 (28) | 21 (22.6) | 0.76 |
| Glass cleaner | 17 (24.3) | 14 (20) | 22 (31.4) | 17 (24.3) | 0.38 |
| Multi-purpose cleaner | 11 (33.3) | 9 (27.3) | 6 (18.2) | 7 (21.2) | 0.52 |
| Degreased spray | 5 (21.7) | 6 (26.1) | 5 (21.7) | 7 (30.4) | 0.88 |
| Air freshener | 5 (20.8) | 2 (8.3) | 9 (37.5) | 8 (33.3) | 0.12 |
| Traditional fume (Esfand) | 32 (25) | 30 (23.4) | 30 (23.4) | 36 (28.1) | 0.20 |
Abbreviation: TSH, thyroid-stimulating hormone.
a Values are expressed as No. (%).
b Data obtained using the chi-square test.
The association between TSH levels and newborn anthropometric characteristics, Apgar scores, and maternal age is presented in Table 4. A significant positive association was observed between TSH and maternal age, and significant negative associations were found between TSH and newborn length and head circumference.
| Variables | TSH | Mother’ s Age | Weight at Birth | Length at Birth | Head Circumference at Birth | 1st-Minute Apgar Score | 5th-Minute Apgar Score |
|---|---|---|---|---|---|---|---|
| TSH | - | 0.16 | -0.09 | -0.20 | -0.22 | 0.04 | 0.05 b |
| Mother age | 0.04 c | - | 0.08 | 0.07 | 0.10 | 0.03 | -0.03 |
| Weight at birth | 0.26 | 0.23 | - | 0.44 | 0.54 | < 0.01 | 0.01 |
| Length at birth | 0.01 c | 0.28 | < 0.001 c | - | 0.34 | -0.07 | 0.01 |
| Head circumference at birth | < 0.01 c | 0.12 | < 0.001 c | < 0.001 c | - | -0.06 | -0.01 |
| 1st-minute Apgar score | 0.54 | 0.56 | 0.95 | 0.24 | 0.32 | - | 0.22 |
| 5th-minute Apgar Score | 0.49 | 0.63 | 0.80 | 0.86 | 0.81 | < 0.01 c | - |
Abbreviation: TSH, thyroid-stimulating hormone.
a The numbers on the upper right part of the table were Spearman’s correlation coefficient, and the numbers on the lower left of the table were their P-values.
b Apgar scores showed no correlation with TSH, possibly due to restricted variability (scores > 8 in 95% of newborns).
c Significant at P < 0.05.
5. Discussion
As the first study of its kind in Iran, we investigated the association between household chemical and green space exposure during pregnancy and cord blood TSH levels. Our results did not show any significant association between these exposures and cord blood TSH levels.
To the best of our knowledge, there are no previous studies specifically addressing the relationship between various types of household chemical exposure during pregnancy and cord blood TSH in neonates. However, over the past decade, several studies have evaluated various classes of thyroid-disrupting chemicals, including industrial chemicals, in pregnant women and their children. These chemicals include polychlorinated biphenyls (PCBs) (16), polybrominated diphenyl ethers (PBDEs) (17), perchlorate (18), bisphenol-A (19), pesticides (20), air pollutants (21), and metals (22).
The results of studies on thyroid-disrupting chemical exposure and neonatal thyroid function have been conflicting. Howe et al. showed that prenatal exposure to particulate matter (PM) in air pollution was associated with higher newborn total T4, especially during early and mid-pregnancy (23). Another study demonstrated a dose-response relationship between air pollutants — particle matter less than 2.5 µm (PM2.5) — and TSH in pregnant women (24). In a study conducted in Iran, exposure to perchlorate during pregnancy was not related to cord blood TSH, T3, or T4 levels in neonates (25). A cohort study suggested that prenatal exposure to some organochlorine compounds may adversely affect thyroid function as evaluated by TSH level at birth (26). However, another study in Belgium showed a significant inverse relationship between organochlorine concentrations and free T3 and free T4, but not with TSH (9).
Findings from a recent study indicated that prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances (PFAS) can act as thyroid-disrupting chemicals and result in neonatal thyroid dysfunction (27). Results from the HOME study in the USA reported that maternal serum PFAS concentrations during the second trimester were not associated with maternal or cord blood thyroid hormone levels (28). Mulder et al. evaluated the association between maternal exposure to organophosphate pesticides during pregnancy and maternal and neonatal cord blood thyroid hormones and found no significant association (29).
Esfand (P. harmala), a traditional fume used by 60.8% of mothers, reflects region-specific practices. Its high exposure (25.12 hours) warrants toxicological investigation.
In this study, for the first time in Iran, we evaluated the relationship between household chemical exposure during pregnancy and cord blood TSH levels; however, no significant association was observed. Our information on exposure was self-reported, and we did not perform laboratory measurements for chemical exposure. Early exposure to certain household chemicals with endocrine-disrupting activity may interfere with neonatal thyroid hormone status, as indicated by some previous studies (9, 20). Thus, further studies are needed to elucidate the patterns of interference. Other confounding factors affecting thyroid hormone status, including other environmental exposures, iodine status, and genetic background, should be considered in future research.
Several studies have examined the effects of green space on a range of health outcomes, such as mortality risk, obesity, prematurity, and mental and developmental health. Liao et al. investigated the association between residential exposure to green space and early childhood neurodevelopment in 1,312 pregnant women and their children in China, suggesting that higher levels of residential green space were associated with better early childhood neurodevelopment (30). Balseviciene et al. found that residential proximity to city parks was associated with lower levels of children’s mental health problems in Spain (31). In another Spanish study, Dadvand et al. reported a positive association between lifelong green space exposure and brain volume development in areas related to working memory and an inverse association with inattentiveness in children (32). A further prospective Spanish cohort study found that increased maternal exposure to surrounding greenness was associated with increased head circumference (33). Although studies on green space and early childhood development are limited, existing research suggests promising benefits of green space exposure on children’s neurodevelopment.
Thyroid hormones in newborns play a critical role in fetal and postnatal neurodevelopment and regulation of neuropsychological function in children (34). Exposure to thyroid-disrupting chemicals during development may have serious consequences, raising the question: "Is the effect of green space on development mediated by thyroid hormones?"
Although previous studies suggest a possible association between TSH levels and green space exposure, our study did not document a significant difference in TSH levels across categories of green space exposure.
Janssen et al. explained that the association between green space exposure and cord blood TSH may be partly explained by reduced levels of traffic-related air pollution, a known endocrine-disruptive factor (21). In a large sample from five cohorts in Europe and the United States, Ghassabian et al. found that first-trimester exposure to PM was associated with mild thyroid dysfunction during pregnancy, while exposure to NOx and NO2 was not associated with hypothyroxinemia or elevated TSH (35). Given the reported association between air pollution and thyroid function (36), it is possible that green space exposure may reduce the risk of adverse effects from air pollutants.
In our study, we did not find a significant association between maternal green space exposure during pregnancy and cord blood thyroid hormones. Unlike Janssen et al. (21), who reported PM2.5-TSH associations, our null findings may reflect differences in exposure type (indoor chemicals versus ambient pollution) or population susceptibility. Cultural and regional differences in exposure patterns may also contribute.
Our study has limitations concerning the accurate evaluation of green space exposure, including the inability to assess exposure quality and the interaction between exposure quality and quantity, as well as the roles of other factors such as air temperature and other indoor and outdoor PM. Further research is needed on the causal relationships between both the quantity and quality of prenatal green space exposure and thyroid hormone levels. Longitudinal Birth cohort studies exploring green space exposure in pregnant women and neurodevelopment in their children, with early evaluation of thyroid function, may provide more conclusive results.
We found a significant direct relationship between TSH and maternal age and significant inverse associations between TSH and newborn length and head circumference. This is consistent with previous studies. Hansen et al. found associations between thyroid hormones and body size, including head circumference, weight, and length at birth, as well as growth from birth to two months (37). This supports the hypothesis that thyroid hormones play a key role in general growth and brain development in healthy children. Another study suggested that thyroid hormones affect fetal growth, while some confounders such as passive maternal smoking are important modifying factors (38). However, Shields et al. found no association between birth measurements and either cord TSH or cord-free T3, but did find an association between cord-free T4 and birth weight, length, and skinfold sum (1). Discrepancies may arise from differences in iodine status, genetic factors, or unmeasured confounders such as maternal diet. Iodine deficiency in Iran (39) could modulate thyroid-growth relationships. There are still conflicting results regarding the association between maternal thyroid function and fetal growth, and the related mechanisms remain to be determined.
The limitations of this study include its relatively small sample size, cross-sectional design, self-reported exposure data, and lack of evaluation of the quality of green space exposure. Unmeasured confounders (e.g., industrial proximity) may influence green space effects. Future studies should incorporate spatial metrics such as NDVI and pollution mapping. The cross-sectional design precludes assessment of temporality; longitudinal studies tracking maternal exposure and child thyroid outcomes are needed. Recall bias in self-reported exposure may result in misclassification of true dose; future studies should combine questionnaires with environmental sensors.
The strengths of this study are its novelty and its focus on a non-Western population. Given the mentioned limitations, future longitudinal studies should include biomarker-based exposure validation to reduce misclassification.
5.1. Conclusions
In this study, exposure to indoor pollutants was relatively high and exposure to green space was low among pregnant women. We did not find a significant association between these exposures and cord blood TSH. However, future longitudinal studies with larger sample sizes and consideration of other confounding factors are necessary to determine potential adverse health effects.
More information on household and environmental pollutant and green space exposure in pregnant women and the growth and neurodevelopmental status of their children, together with early evaluation of thyroid function, may provide more conclusive findings.