1. Background
Schizophrenia is a chronic psychotic disorder characterized by disturbances in thought processes, emotions, and behaviours (1). Patients often exhibit illogical thinking, misperceptions, and impaired attention, and may withdraw into hallucinations (1, 2). Globally, schizophrenia is recognized as a major cause of disability that significantly affects individuals’ ability to function in educational, occupational, and social settings (3).
According to the World Health Organization, approximately 20 million people are affected by schizophrenia worldwide. In Indonesia, data from the 2018 National Basic Health Research (RISKESDAS) indicated that around 400,000 people live with schizophrenia, with the majority residing in community settings rather than in mental health institutions (4). This condition poses a considerable public health challenge, requiring long-term management strategies that effectively reduce symptoms and improve quality of life (3, 5-7).
Paranoid schizophrenia is the most common subtype, marked by prominent delusions and auditory hallucinations (8). Second-generation antipsychotics like risperidone are frequently used as first-line therapy. However, in treatment-resistant cases, clozapine is often introduced as an add-on strategy to enhance symptom control. At Dr. H. B. Saanin Psychiatric Hospital in Padang, both risperidone monotherapy and risperidone-clozapine combination therapy are commonly prescribed, yet data on their comparative effectiveness in symptom reduction remain limited (2, 9, 10).
2. Objectives
This study aimed to compare the effects of risperidone monotherapy versus risperidone plus clozapine add-on therapy on agitation symptoms in patients with paranoid schizophrenia.
3. Methods
3.1. Study Design and Setting
This was a retrospective observational quasi-experimental study conducted at Dr. H. B. Saanin Mental Hospital, Padang, Indonesia.
3.2. Participants
Patients aged 18 - 65 years diagnosed with paranoid schizophrenia (ICD-10 F20.0) (11) were eligible if they had received risperidone monotherapy or risperidone plus clozapine add-on therapy for at least eight weeks, including symptom assessment using the Positive and Negative Syndrome Scale-Excited Component (PANSS-EC) (12, 13). The duration of illness at the time of study entry was recorded for those newly diagnosed or with a prior history of schizophrenia. Patients with comorbid psychiatric diagnoses or severe medical illnesses were excluded. Patients were not excluded based on the use of other psychotropic medications, which were prescribed as part of routine care.
3.3. Variables
The primary outcome was symptom severity measured using the PANSS-EC. The main exposure variable was the type of antipsychotic therapy: Risperidone monotherapy or risperidone-clozapine combination. Sociodemographic and clinical characteristics (age, gender, education, duration of illness, and treatment) were also collected.
3.4. Treatment Allocation
In practice, all patients first received risperidone monotherapy. Clozapine was added later by psychiatrists for treatment-resistant cases who showed insufficient response to risperidone. This decision was made according to national guidelines and clinical judgment.
3.5. Data Sources and Outcome Measurement
Data were obtained from patient medical records and hospital databases. The primary outcome was change in agitation, measured using the PANSS-EC Indonesian version, a validated instrument for assessing agitation and excitement-related symptoms. Psychometric validation studies in Indonesian populations confirm its reliability. The PANSS-EC scores were extracted from clinical assessments documented by psychiatrists.
3.6. Bias
All eligible patients within the study period were included through consecutive sampling to reduce selection bias. Information bias was minimized by using validated instruments (PANSS-EC), with the license number 6046840588742 (14), and cross-checking records by two independent researchers (NF and YOS). Patients were assigned to treatment groups based on their prescribed therapy during routine clinical care; therefore, matching procedures were not applicable. To reduce confounding, statistical tests were used to compare baseline characteristics between the two groups and ensure comparability.
3.7. Study Size
From January to December 2023, a total of 47 patients met the inclusion criteria. A formal sample size calculation was not performed due to consecutive sampling.
3.8. Quantitative Variables and Statistical Methods
Descriptive statistics were used to summarize demographic and clinical data. Group differences were analyzed using the chi-square test (15) for categorical variables and the Mann-Whitney test for non-normally distributed continuous variables (16). The Kruskal-Wallis test was used to compare Positive and Negative Syndrome Scale (PANSS) subscale scores across therapy groups. A level of P < 0.05 was considered statistically significant. All analyses were performed using SPSS version 25.0®. Differences in clinical outcomes, including PANSS-EC scores and length of hospital stay, were compared between these subgroups using the Mann-Whitney test for continuous variables and the chi-square test for categorical variables.
4. Results
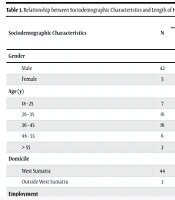
During the study, a total of 410 inpatients were diagnosed with paranoid schizophrenia at Dr. H. B. Saanin Mental Hospital. Of the 47 patients, 38 received risperidone monotherapy and 9 received risperidone plus clozapine (Table 1). Both groups showed PANSS-EC score reductions. The risperidone group had a mean reduction of 4.26 ± 3.20, while the add-on group had a slightly higher reduction, although not statistically significant. The mean length of stay (LoS) was 20.95 ± 6.18 days for risperidone monotherapy and 20.00 ± 4.69 days for the combination group (P = 0.878). Table 2 displays the differences in PANSS-EC scores according to sociodemographic characteristics, while Table 3 shows the relationship between these characteristics and the patients' length of hospitalization. No significant associations were found between sociodemographic characteristics and changes in agitation scores.
| Characteristics | Risperidone | Risperidone + Clozapine | Total Patients | P-Value |
|---|---|---|---|---|
| Gender | 0.210 b | |||
| Male | 35 (92.1) | 7 (77.8) | 42 (89.4) | |
| Female | 3 (7.9) | 2 (22.2) | 5 (10.6) | |
| Age (y) | 0.312 c | |||
| 18 - 25 | 7 (18.4) | 0 (0) | 7 (14.9) | |
| 26 - 35 | 12 (31.6) | 4 (44.4) | 16 (34) | |
| 36 - 45 | 13 (34.2) | 3 (33.3) | 16 (34) | |
| 46 - 55 | 5 (13.2) | 1 (11.1) | 6 (12.8) | |
| > 55 | 1 (2.6) | 1 (11.1) | 2 (4.3) | |
| Domicile | 0.519 b | |||
| West Sumatra | 36 (94.7) | 8 (88.9) | 44 (93.6) | |
| Outside West Sumatra | 2 (5.3) | 1 (11.1) | 3 (6.4) | |
| Employment | 0.053 b | |||
| Employed | 12 (31.6) | 0 (0) | 12 (25.5) | |
| Unemployed | 26 (68.4) | 9 (100) | 35 (74.5) | |
| Education level | 0.498 c | |||
| Elementary school | 14 (36.8) | 4 (44.4) | 18 (38.3) | |
| Junior high school | 11 (28.9) | 3 (33.3) | 14 (29.8) | |
| Senior high school | 12 (31.6) | 2 (22.2) | 14 (29.8) | |
| Bachelor’s degree | 1 (2.6) | 0 (0) | 1 (2.1) |
a Values are expressed as No. (%).
b Chi-square.
c Fisher’s exact test.
| Sociodemographic Characteristics | N | PANSS-EC Scores Differences | P-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | CI 95% | ||||
| Lower - Upper | Min - Max | ||||
| Gender | 0.221 a | ||||
| Male | 42 | 4.31 ± 3.475 | 3.23 - 5.39 | 0 - 15 | |
| Female | 5 | 5.4 ± 2.191 | 2.68 - 8.12 | 3 - 9 | |
| Age (y) | 0.337 b | ||||
| 18 - 25 | 7 | 4.71 ± 2.928 | 2.01 - 7.42 | 1 - 9 | |
| 26 - 35 | 16 | 4.56 ± 3.521 | 2.69 - 6.44 | 1 - 14 | |
| 36 - 45 | 16 | 3.81 ± 3.728 | 1.83 - 5.8 | 0 - 11 | |
| 46 - 55 | 6 | 6 ± 2.966 | 2.89 - 9.11 | 2 - 11 | |
| > 55 | 2 | 2.5 ± 0.707 | -3.85 - 8.85 | 2 - 3 | |
| Domicile | 0.947 a | ||||
| West Sumatra | 44 | 4.45 ± 3.45 | 3.41 - 5.5 | 0 - 15 | |
| Outside West Sumatra | 3 | 4 ± 2 | -0.97 - 8.97 | 2 - 6 | |
| Employment | 0.561 a | ||||
| Employed | 12 | 4.92 ± 3.895 | 2.44 - 7.39 | 0 - 15 | |
| Unemployed | 35 | 4.26 ± 3.2 | 3.16 - 5.36 | 0 - 14 | |
| Education level | 0.423 b | ||||
| Elementary school | 18 | 4.22 ± 3.191 | 2.64 - 5.81 | 1 - 15 | |
| Junior high school | 14 | 4.64 ± 2.205 | 3.37 - 5.92 | 1 - 9 | |
| Senior high school | 14 | 4.71 ± 4.548 | 2.09 - 7.34 | 0 - 14 | |
| Bachelor's degree | 1 | 0 | 0 | 0 | |
Abbreviation: PANSS-EC, Positive and Negative Syndrome Scale-Excited Component.
a Mann Whitney.
b Kruskal Walis.
| Sociodemographic Characteristics | N | LoS | P-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | CI 95 % | ||||
| Lower - Upper | Min - Max | ||||
| Gender | 0.464 a | ||||
| Male | 42 | 20.95 ± 6.18 | 19.03 - 22.88 | 2 - 35 | |
| Female | 5 | 20.00 ± 4.69 | 14.18 - 25.82 | 15 - 27 | |
| Age (y) | 0.404 b | ||||
| 18 - 25 | 7 | 20 ± 4,726 | 15,63 - 24,37 | 15 - 28 | |
| 26 - 35 | 16 | 18,81 ± 7,985 | 14,56 - 23,07 | 2 - 32 | |
| 36 - 45 | 16 | 23,19 ± 4,875 | 20,59 - 25,79 | 18 - 35 | |
| 46 - 55 | 6 | 21,17 ± 3,251 | 17,76 - 24,58 | 17 - 27 | |
| > 55 | 2 | 20,5 ± 0,707 | 14,15 - 26,85 | 20 - 21 | |
| Domicile | 0.809 a | ||||
| West Sumatra | 44 | 20.37 ± 6.11 | 18.87 - 22.59 | 2 - 35 | |
| Outside West Sumatra | 3 | 22.67 ± 4.62 | 11.19 - 34.14 | 20 - 28 | |
| Employment | 0.834 a | ||||
| Employed | 12 | 21.17 ± 3.09 | 19.2 - 23.14 | 15 - 28 | |
| Unemployed | 35 | 20.74 ± 6.76 | 18.42 - 23.06 | 2 - 35 | |
| Education level | 0.347 b | ||||
| Elementary school | 18 | 21.06 ± 3.539 | 19.3 - 22.82 | 15 - 32 | |
| Junior high school | 14 | 20.36 ± 4.94 | 17.51 - 23.21 | 11 - 28 | |
| Senior high school | 14 | 20.07 ± 8.398 | 15.22 - 24.92 | 2 - 33 | |
| Bachelor's degree | 1 | 0 | 0 | 0 | |
Abbreviation: LoS, length of stay.
a Mann Whitney.
b Kruskal Walis.
5. Discussion
This study compared risperidone monotherapy with clozapine add-on therapy for agitation symptoms in paranoid schizophrenia. Although the combination group showed a trend toward greater improvement, the difference was not statistically significant. The findings align with previous evidence that clozapine may provide additional benefit in treatment-resistant cases but require larger studies to confirm.
Analysis of sociodemographic characteristics in relation to PANSS-EC score differences revealed no statistically significant associations. The Mann-Whitney test showed no significant relationship between gender and PANSS-EC score changes (P = 0.337), although the mean score was higher in females (5.4) compared to males (4.31). This aligns with He's and Flynn’s findings, who noted that male patients often present with lower PANSS scores (17, 18). Similarly, age, residence, employment status, and education level were not significantly associated with PANSS-EC score differences (P > 0.05). Among the age groups, the highest mean difference was observed in the 46 - 55 age group (mean = 6), while the lowest was in the > 55 group (mean = 2.5).
Regarding the LoS, statistical analyses using Mann-Whitney and Kruskal-Wallis indicated no significant influence of gender (P = 0.464), age (P = 0.404), residence (P = 0.809), employment (P = 0.834), or education level (P = 0.347). However, female patients tended to have shorter LoS than males. The highest mean LoS was found in the 36 - 45 age group (23.19 days). It has been reported that older patients may experience longer hospitalizations due to reduced social support (1, 19). Gender differences in schizophrenia prognosis have been attributed to the neuroprotective effects of estrogen (20-23). Estrogen can inhibit dopamine release in brain areas such as the nucleus accumbens, where dopamine receptor dysregulation is implicated in schizophrenia (20, 24). This hormonal protection may explain the better outcomes observed in females. Meta-analyses show schizophrenia is 1.5 times more prevalent in males, who also tend to exhibit more negative symptoms than females (25, 26).
Although education is known to improve patients’ knowledge and insight into schizophrenia (26), this study found no significant association between education level and LoS (27-29). Patients with severe psychiatric disorders often experience impaired social functioning, which can affect recovery (3, 9, 30). Furthermore, 75% of patients with severe schizophrenia are typically unemployed. Though most sociodemographic variables were not significantly related to LoS or symptom improvement, previous research suggests that cultural and social factors may play a role. For example, a study in Ethiopia found that migrants from culturally distinct backgrounds had a higher risk of developing schizophrenia (31). The current study found no significant associations between LoS and educational level, occupation, or patient type (new vs. returning) (28, 32). However, Table 3 indicated substantial associations between LoS and employment (P = 0.003) and patient visit status (P = 0.005). There was no significant relationship between the type of pharmacological therapy (monotherapy vs. combination) and LoS (P = 0.878). Even though clozapine typically requires longer titration and delayed onset of efficacy compared to other antipsychotics, this might offset its impact on reducing LoS in the short term (33).
Recent work has shown that augmentation strategies may enhance antipsychotic treatment outcomes, such as modafinil added to risperidone or olanzapine for improving persistent symptoms in schizophrenia (34). In addition, cognitive impairment — an important clinical dimension in schizophrenia — has been linked to treatment response and overall symptom dynamics, including behavioral manifestations such as agitation (35). These findings support the concept that individualized augmentation approaches may be beneficial when monotherapy is insufficient. Our study aligns with this perspective, showing that clozapine add-on therapy may offer advantages in managing agitation in selected patients.
5.1. Conclusions
Clozapine add-on to risperidone showed a non-significant trend toward greater reduction in agitation compared to risperidone monotherapy. However, the small and unequal group sizes, lack of dosage and adherence data, and short follow-up period limit the generalizability of these findings. Larger, prospective, and multi-center studies with comprehensive outcome measures are recommended to validate and expand on these results.
5.2. Strengths and Limitations
This study has notable strengths. Conducted in a real clinical setting, the findings are directly relevant to psychiatric practice. Comparing risperidone monotherapy with clozapine add-on therapy provides valuable insight into treatment strategies for schizophrenia. The use of the standardized PANSS-EC Scale ensured objective assessment of agitation, and inclusion of sociodemographic factors broadened understanding of influences on treatment response and hospital stay.
Several limitations should be acknowledged. The sample size was small and uneven (38 vs. 9), limiting statistical power and generalizability. Conducted at a single hospital, results may not apply to other settings. Dosage information was inconsistently documented, and adherence was not systematically assessed. Other potential confounders, such as illness duration, family support, and substance use, were not captured. The retrospective design prevents causal inference. Outcomes were limited to hospitalization, so long-term effects such as relapse and functional recovery remained unknown. The PANSS-EC assessed only agitation, not the full spectrum of schizophrenia symptoms. Finally, as only inpatients were included, findings may not represent those with milder disease managed in outpatient care. Future research should be larger, multi-center, and prospective, with balanced groups, dosage and adherence data, broader measures, and longer follow-up.